

Microbial Degradation of Environmental Microplastics
†These authors contributed equally to this work.
Received date: 2024-07-10
Revised date: 2024-09-29
Online published: 2024-12-25
Supported by
National Natural Science Foundation of China(22241603)
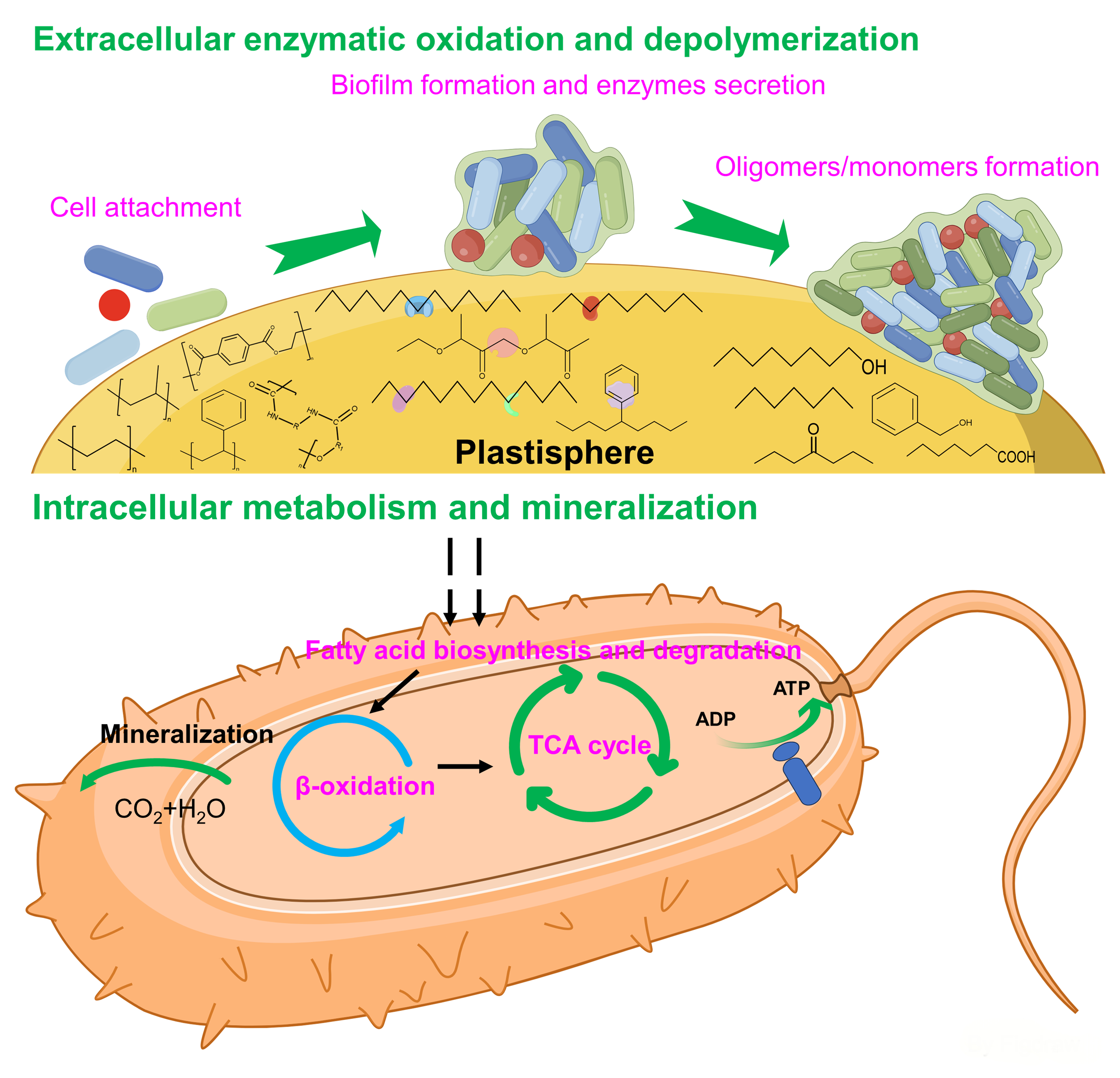
Due to the highly stable chemical properties of plastics, plastic wastes disposed into environments are difficult to degrade and can only be broken down into microplastics with smaller particle size and larger surface area through the weathering process. Microplastic pollution has become one of the most pressing environmental issues. There is an urgent need to reduce microplastic pollution in order to protect the ecological and human health. Biodegradation of microplastics can ultimately convert microplastics into environmentally friendly substances such as biomass, CO2, CH4 and H2O or other valuable intermediates. It is thus an environmentally friendly technology to potentially make microplastics harmless and resourceful. This paper reviews the present understanding of microplastics biodegradation processes, the influencing factors, the microbial and enzymatic resources for microplastics degradation, and the up-to-date approaches for mining plastics-degrading microbial resources. It finally provides perspectives on the challenges of current research and the direction of future research on microplastic biodegradation.
1 Introduction
2 Microplastic biodegradation process
2.1 Degradation pathway
2.2 Influence factors
3 Microplastic biodegradation resources
3.1 Degrading bacteria
3.2 Catabolic enzymes
3.3 Synthetic community
4 Mining strategies for microplastics-degrading microorganisms
4.1 Culture-dependent methods
4.2 Culture-independent methods
5 Conclusion and outlook
Hongqin Guo , Kai Yang , Li Cui . Microbial Degradation of Environmental Microplastics[J]. Progress in Chemistry, 2025 , 37(1) : 112 -123 . DOI: 10.7536/PC240706
| Polymers | Abbreviation | Molecular formular | Backbone structure | Estimated half-lives ( year) |
|---|---|---|---|---|
| polystyrene | PS | (C8H8)n | 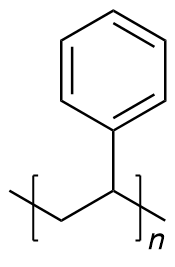 | >2500 |
| polyethylene | PE | (C2H4)n | 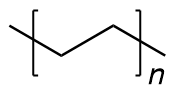 | 58~5000 |
| polypropylene | PP | (C3H6)n | 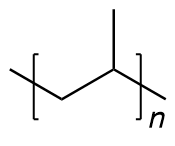 | 53~780 |
| polyethylene terephthalate | PET | (C10H8O4)n | 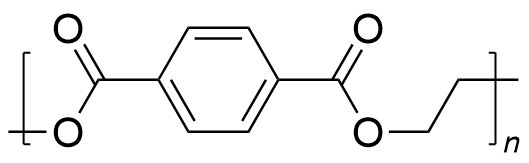 | 2.3~2500 |
| polyvinyl chloride | PVC | (C2H3Cl)n | 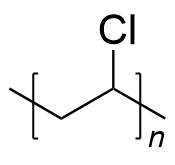 | >2500 |
| polyurethane | PU | (NHCOO)n | 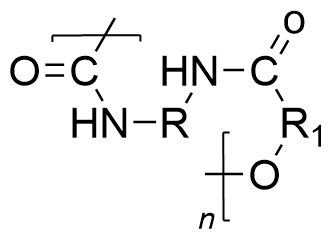 | 10~999 |
| polycaprolactone | PCL | (C6H10O2)n | 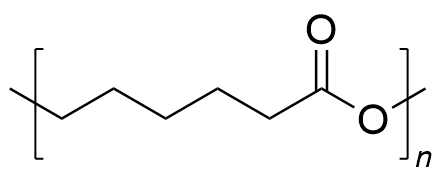 | 0.19~3.1 |
| polylactic acid | PLA | (C3H4O2)n | 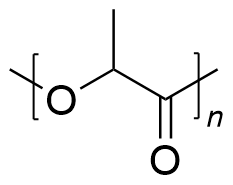 | 0.19~3.1 |
| polyhydroxyalkanoates | PHA | (C5H8O2)n | 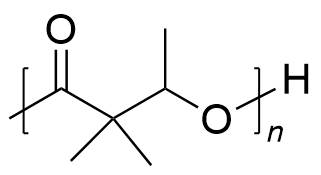 | 0.19~3.1 |
| polyvinyl alcohol | PVA | (C2H4O2)n | 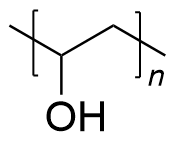 | 0.19~3.1 |
| polyester | PES | (C6H10O5)n | 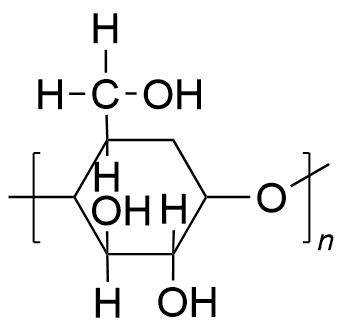 | 0.19~3.1 |
| polycarbonate | PC | (C16H18O5)n | 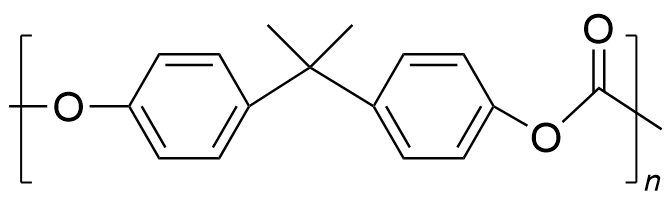 | 0.19~3.1 |
| polyhydroxybutyrate | PHB | (C4H6O2)nH2O | 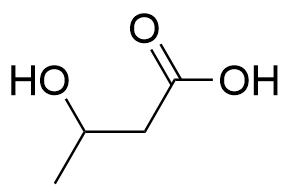 | 0.19~3.1 |
| polyamide | PA | (C6H11NO)n | 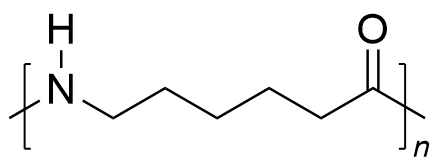 | 0.19~3.1 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
UNEP(2014) Plastic debris in the ocean. UNEP Year Book 2014 Emerging issues update. [2024-11-24] https://www.unep.org/resources/report/unep-year-book-2014-emerging-issues-our-global-environment.
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
(李昕玥, 刘卓苗, 薛润泽, 代燕辉, 岳同涛, 赵建. 科学通报, 2021, 66 (20): 2573. )
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
Zeenat.
|
| [35] |
|
| [36] |
(郭鸿钦, 罗丽萍, 杨宇航, 王宇萌, 陆遥力, 赵鑫, 胡筱敏. 应用与环境生物学报, 2020, 26 (06): 1546. ).
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
Sankara Subramanian S H,
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
/
| 〈 |
|
〉 |