

Neurotoxicity and Mechanisms of Microplastics and Nanoplastics Exposure in Terrestrial Mammals
†These authors contributed equally to this work.
Received date: 2024-07-10
Revised date: 2024-12-18
Online published: 2025-01-07
Supported by
National Natural Science Foundation of China(82273656)
National Natural Science Foundation of China(82304177)
National Natural Science Foundation of China(22241604)
National Natural Science Foundation of China(82073519)
Guangdong Province University Student Innovation and Entrepreneurship Training Program projects(S202312121124)
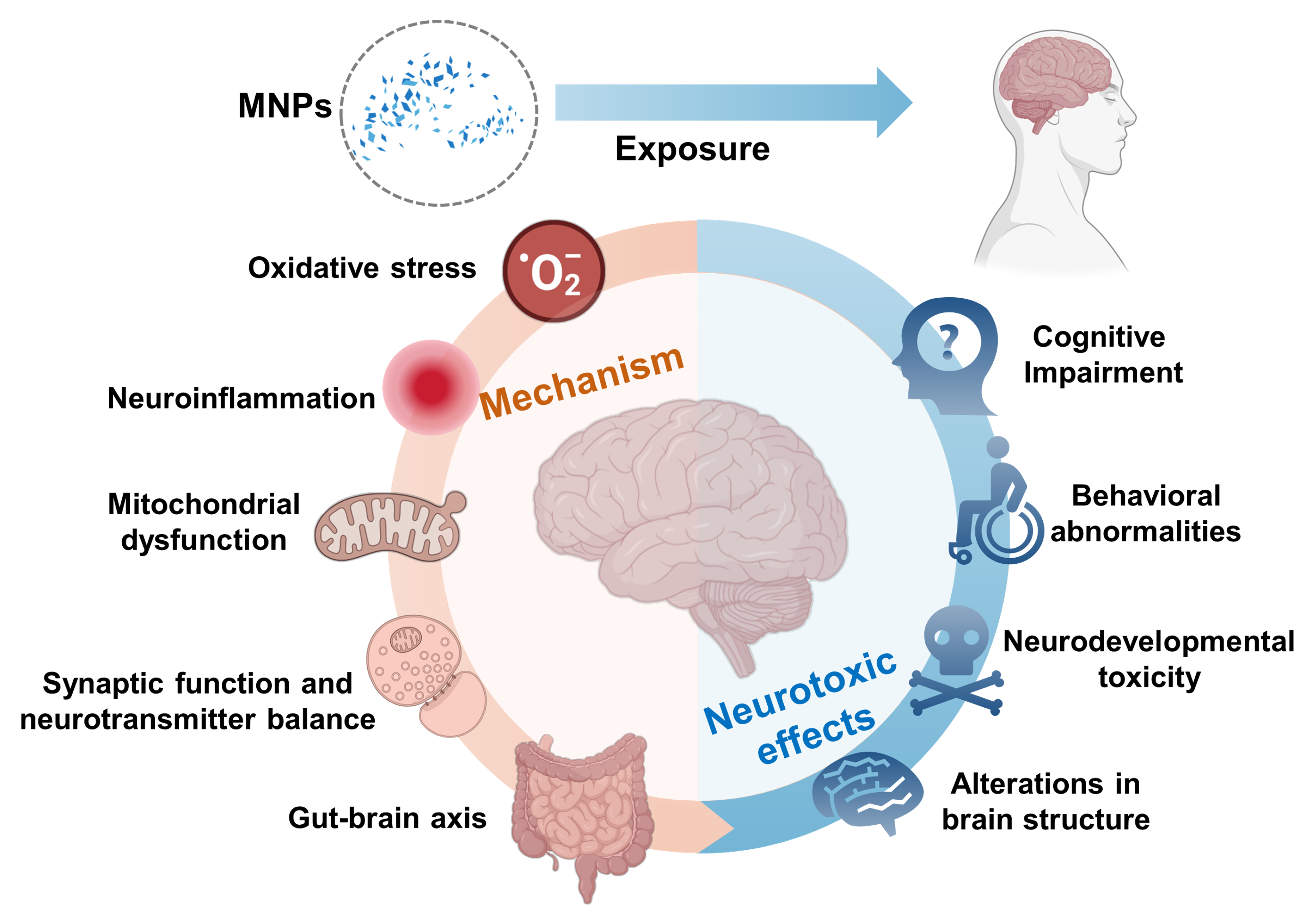
Microplastics and nanoplastics (MNPs) pollution has become a serious environmental issue. MNPs can enter the human body through inhalation, ingestion, and skin contact, raising significant concerns about their potential risks to the nervous system. This paper reviews the studies on the neurotoxic effects of MNPs in terrestrial mammals, focusing on their possible toxic mechanisms. Studies have shown that MNPs can cause damage to the nervous system by inducing oxidative stress, inflammatory responses, and mitochondrial dysfunction. Additionally, the impact of MNPs on the gut-brain axis is considered a crucial mechanism leading to neurotoxicity. Despite current progress, there are still insufficient data and incomplete understanding of the neurotoxic mechanisms involved. Future research should enhance epidemiological studies on MNP exposure, develop more suitable experimental models, investigate the health effects of different types of MNPs, explore their mechanisms in greater depth, and comprehensively assess various influencing factors. These efforts will provide essential insights for a more thorough understanding of the impact of MNPs on human health.
1 Introduction
2 Human exposure to MNPs
2.1 Routes of human exposure to MNPs
2.2 Detection of MNPs in human tissues and organs
3 Neurotoxic effects of MNPs
3.1 Cognitive impairment
3.2 Behavioral abnormalities
3.3 Neurodevelopmental toxicity
3.4 Alterations in brain structure
3.5 Combined neurotoxic effects of MNPs and other environmental pollutants
4 Mechanisms of toxicity
4.1 Oxidative stress
4.2 Neuroinflammation
4.3 Mitochondrial dysfunction
4.4 Synaptic function and neurotransmitter balance
4.5 Gut-brain axis mechanism
5 Conclusion and outlook
Key words: microplastics and nanoplastics; nervous system; mammals; toxic mechanisms
Yuji Huang , Kailin Xu , Boxuan Liang , Yizhou Zhong , Maoyong Song , Zhenlie Huang . Neurotoxicity and Mechanisms of Microplastics and Nanoplastics Exposure in Terrestrial Mammals[J]. Progress in Chemistry, 2025 , 37(1) : 76 -88 . DOI: 10.7536/PC240709
Figure 1 Distribution of micro nano plastics in human tissues and organsFig. 1 Distribution of MNPs in the human body |
Table 1 Distribution of MNPs in human tissues and organsTable 1 MNPs were detected in various parts of the human body |
| Human tissue types | MNPs types | MNPs content range | Ref |
|---|---|---|---|
| Blood | PET, PVC, PP, PMMA | 1.6 μg/mL | 39 |
| Vitreous humor | PA66, PVC, PS | 0~15.7 μg/g | 44 |
| Hairs | PP, PET, PS | 3.5 MPs/day | 17 |
| Liver | PS, PVC, PET | 0~1.5 MPs/g | 40 |
| Kidney | PS, PVC, PET | 0~0.3 MPs/g | 40 |
| Spleen | PS, PVC, PET | 0.4~2.2 MPs/g | 40 |
| Colon | PS, PVC, PET | 0~0.3 MPs/g | 41 |
| Lung | PP, PET, PS | 0.56 MPs/g | 42 |
| Placenta | PVC, PP, PBC | 0.29~9.55 MPs/g | 43 |
Table 2 Study on the neurotoxicity of micro nano plastic exposure to terrestrial mammalsTable 2 Research on the neurotoxicity of MNP exposure in terrestrial mammals |
| Species | MNPs types | Size | Method of administration | Exposure dose | Exposure duration | Neurotoxic effects | Ref |
|---|---|---|---|---|---|---|---|
| Male wistar rats (6~8 weeks) | PS-NPs | 25 and 50 nm | Oral gavage | 1,3,6,and 10 mg/(kg·d) | 35 d | No significant neurobehavioral effects. | Rafiee et al. [47] |
| Male BALB/c mice (6 weeks) | PS-MPs | 0.5,4 and 10 μm | Drinking water exposure | 100 and 1000 μg/L | 180 d | Cognitive and memory deficits; disruption of the blood-brain barrier; reduced dendritic spine density; an inflammatory response in the hippocampus; a concentration-dependent trend. | Jin et al. [48] |
| Male C57BL/6 mice (5 weeks) | PS-NPs | 25 nm | Oral gavage | 0,10,25, 50 mg/(kg·d) | 180 d | Cognitive decline; synaptic damage in the prefrontal cortex; increased sensitivity to synaptic dysfunction. | Chen et al. [49] |
| Male C57BL/6 mice (5 weeks) | PS-NPs | 80 nm | Oral gavage | 60 μg/day | 42 d | Caused neuronal damage in the hippocampus,impaired learning and memory abilities; neurotoxicity involved gut-brain axis-mediated pathways related to circadian rhythm. | Kang et al. [50] |
| Male C57BL/6 mice (7 weeks) | PS-NPs | 30~50 nm | Oral gavage | 10 and 20 mg/(kg·d) | 42 d | Induced memory impairment,activated microglia,triggered inflammatory responses,and dysregulated hippocampal neuronal activity. | Paing et al. [51] |
| Male Swiss mice (4~6 weeks) | PS-NPs | 25 nm | Intraperitoneal administration | 14.6 ng/(kg·d) | 3 d | Cognitive impairment; REDOX imbalance; suppressed AChE activity; DNA damage. | Estrela et al. [52] |
| Male or female C57BL/6 mice (6 weeks) | PS-MPs | 2 μm | Oral gavage | 8 and 16 mg/(kg·d) | 28 and 56 d | Learning and memory deficits; decreased synaptic protein levels; induced neuroinflammation. | Lee et al. [53] |
| SH-SY5Y cells | Amine-functionaliz- ed PS-NPs | 50 nm | In culture medium | 1, 5, 10, and 50 µg/cm2 | 24 h and 48 h | Decreased neuronal differentiation and increased Amyloid β secretion. | Schroter et al. [54] |
| SHSY-5Y cells | Unmodified and amino-modified PS-NPs | 70 and 150 nm | In culture medium | 100 pM | 24 h and 7 d | Accelerated the aggregation of β-amyloid peptides. | Gou et al. [55] |
| Male C57BL/6 mice (6~8 weeks) | PS-MPs and PS-NPs | 100 nm and 1.0 µm | Oral gavage | 0.5 mg/day | 30 and 60 d | Induced anxiety-like behaviors; changed serum metabolic profiles,particularly in neurotransmitter metabolites. | Chen et al. [56] |
| Male C57BL/6 mice (8 weeks) | PS-MPs | 480 nm | Oral gavage | 2 and 10 mg/(kg·d) | 180 d | Induced anxiety-like behavior; related to inflammation pathways. | Li et al. [57] |
| Male C57BL/6 mice (5 weeks) | PS-NPs | 25 nm | Oral gavage | 10, 25 and 50 mg/(kg·d) | 180 d | Depression-like responses; activation of axon guidance,the neurotrophin signaling pathway,and dopaminergic synapse-related lncRNAs. | Liu et al. [58] |
| Male BALB/c mice (4 weeks) | PS-NPs with different surface modifications (PS,PS-COOH,and PS-NH2) | ∼100 nm | Oral gavage | 1 mg/day | 28 d | Disrupted the tight junctions formed by brain endothelial cells; induced mitochondrial dysfunction in neurons; induced anxiety,depression,and social deficits. | Ma et al. [59] |
| Male Swiss albino mice (10 weeks) | PS-NPs | 50 nm | Oral gavage | 0.2 and 1 mg/(kg·d) | 56 d | Induced anxiogenic behavior and impaired learning; induced oxidative stress and inhibited acetylcholinesterase activity; decreased the number of hippocampal pyramidal cells; affected neuronal architecture of the cortex and hippocampus. | Sharma et al. [60] |
| Male C57BL/6 mice (8 weeks) | PS-NPs | 50 nm | Oral gavage | 0.5, 5, 50 and 500 mg/(kg·d) | 28 d | Induced Parkinson’s disease (PD)-like neurodegeneration; disturbed PD-associated gene expression; inhibited ATP metabolism | Liang et al. [61] |
| Male C57BL/6 mice (8 weeks) and SHSY-5Y cells | PS-NPs | 50 nm | in vivo:Oral gavag; in vitro: in culture medium | in vivo: 500 mg/(kg·d) ; in vitro: 0.25,2.5, 25, 250 μg/mL | in vivo: 28 d in vitro: 48 h | PS-NPs disrupted mitochondrial function by affecting CI,leading to excessive mitophagy through the AMPK/ULK1 pathway,ultimately causing dopaminergic neuron death. | Huang et al. [62] |
| Primary neuron; CD1 mice (3 months) | Anionic and cationic PS-NPs | 23.2~ 115.6 nm | in vivo: intracranial injection; in vitro: in culture medium | in vivo: 15 μg; in vitro: 1 nM | in vivo: 3-d, or two months; in vitro: 24 h | Causing mild lysosomal impairment that slows the degradation of aggregated α-synuclein; exacerbates the spread of α-synuclein pathology across interconnected vulnerable brain regions. | Liu et al. [65] |
| C. elegans strains; A53T α-syn-EGFP SH-SY5Y cells | PS-NPs | 25 nm | in vivo: in culture medium; in vitro: in culture medium | in vivo: 10,100,or 1000 μg/L; in vitro: 15 mg/L | in vivo: 24 h; in vitro: 24 h | Low concentrations of nanoplastics inhibit the growth and movement of nematodes; increase α-synuclein aggregation. | Jeong et al. [66] |
| SD pregnant rats | PS-NPs | 25 and 50 nm | Oral gavage | 0.5, 2.5, 10 and 50 mg/(kg·d) | 1st to the 18th day of pregnancy | Affect the growth and development of fetal rats; damage the placental barrier; cause oxidative stress and inflammatory responses. | Zhang et al. [68] |
| Pregnant female C57BL/6 mice | PS-NPs | 50 and 500 nm | Oral administration | 0.5~1000 μg/day | embryonic day 8 until 2 weeks after birth | Induced neurodevelopmental defects; caused NSC dysfunction; led to the dysregulation of brain functions in progeny. | Jeong et al. [69] |
| 3D model of human forebrain cortical spheroids | PS-MPs | 1 and 10 μm | In culture medium | 5,50,and 100 µg/mL | 6 and 26 d | Altered the gene expression related to DNA damage and neural tissue patterning; affected embryonic brain-like tissue development in forebrain cerebral spheroids. | Hua et al. [71] |
| C57BL/6 J mice (8-weeks) | PS-MPs | 2 μm | Drinking water exposure | 1 mg/L | embryonic stage to adulthood | Impaired offspring social novelty preferences. | So et al. [73] |
| Female C57BL/6 J mice | PE-MPs | 10~20 μm | Oral gavage | 10 μg/day | prenatal: 14 d; post-weaning period: 14 d; puberty: 14 d; adult: 84 d. | Impaired social interaction and repetitive behaviors; disturbance of metabolites and gene expression in the brain; leads to Autism Spectrum Disorder (ASD)-like traits. | Zaheer et al. [74] |
| Male C57BL/6 mice (8 weeks); hCMEC/D3,murine microglia BV2,hippocampus-derived neuronal HT22 cells | PS-NPs | 42 nm | in vivo: Oral gavage; in vitro: in culture medium | in vivo: 0.5,2.5,10,and 50 mg/(kg·d); in vitro: 0,25,50,and 100 μg/mL | in vivo: 7 d; in vitro: 48 h | Induced microglia activation and neuronal damage in the brain; caused oxidative stress,inflammatory response,and necroptosis in hCMEC/D3 cells; disrupted tight junctions. | Shan et al. [76] |
| Male C57BL/6 mice (3 weeks) | PS-MPs | 5 and 50 μm | Drinking water exposure | 100 and 1000 μg/L | 70 d | Induced social disorders in adult mice; decreased oxytocin levels; reduced mucin layers and caused gut microbiota dysbiosis. | Wang et al. [77] |
| Male Swiss albino mice (20~30 g) | PS-MPs | 500 nm | Oral gavage | 0.1,1,and 10 ppm | 28 d | Reduced Nissl bodies in the PFC; affected neuronal cytoarchitecture of PFC neurons; decreased spine density in PFC neurons; down-regulated BDNF gene. | Suman et al. [79] |
| Male KM mice (5 weeks) | PS-MPs | 5.0~5.9 μm | Oral gavage | 1 mg/d | 28 d | Impaired learning and memory functions; induced a reduction in the level of acetylcholine; increased levels of ROS and MDA. | Wang et al. [80] |
| KM mice (18~22 g); NS20Y cells | PS-MPs; Di-(2-ethylhexyl) phthalate (DHEP) | 1~10 μm | Food and water exposure | in vivo: food containing 200 mg/kg DEHP and water containing 10 mg/L MPs; in vitro: 25 μmol/L DEHP and 775 mg/L PS-MPs | in vivo: 30 d; in vitro: 24 h | DEHP and MPs caused changes in mitochondrial function; led to apoptosis of neurons; exhibited a combined toxic effect on mouse cerebra. | Zhang et al. [82] |
| Male ICR mice (3 weeks) | PP-MPs; DHEP | / | Oral gavage | PP-MPs: 5,20 and 80 mg/(kg·d); DHEP: 250 mg/(kg·d) | 28 d | Neurocognitive defects; induced impairments in the hippocampal CA3 region; exhibited additive or synergistic toxic effects. | Yang et al. [83] |
| Male C57BL/6 mice (7~8 weeks) | PS-MPs; doxycycline hyclate (Dox) | 500 nm | Oral gavage | PS-MPs: 5 mg/(kg·d); Dox: 20 mg/(kg·d) | 30 d | Co-exposure caused brain lesions and inflammation; decreased learning and memory behaviors through the gut-brain axis. | Sun et al. [84] |
| Male C57BL/6 mice (9 months) | PS-MPs; Ferric ammonium citrate (FAC) | 5 µm | Drinking water exposure | PS-MPs: 1000 μg/L; Dox: 5 g/L | 90 d | Co-exposure caused significant iron overload and cognitive deficits; elevated lipid peroxidation and inflammation; aggravated cognitive impairment. | Liu et al. [85] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
(杨壹珺, 王帅兵, 叶长兵, 史应仙, 李雪柔, 原祎昕, 霍佳祺. 环境保护前沿, 2024, 14:51. ).
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
(张亚萍, 田蕾, 谢晓倩, 王亚婷, 吕鹏, 袭著革. 中国应用生理学杂志, 2022, 38(06):760.)
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
/
| 〈 |
|
〉 |