

Extraction Technology of Liquid Lithium
Received date: 2024-02-26
Revised date: 2024-08-11
Online published: 2024-08-30
Supported by
National Natural Science Foundation of China(42077299)
National Natural Science Foundation of China(42025704)
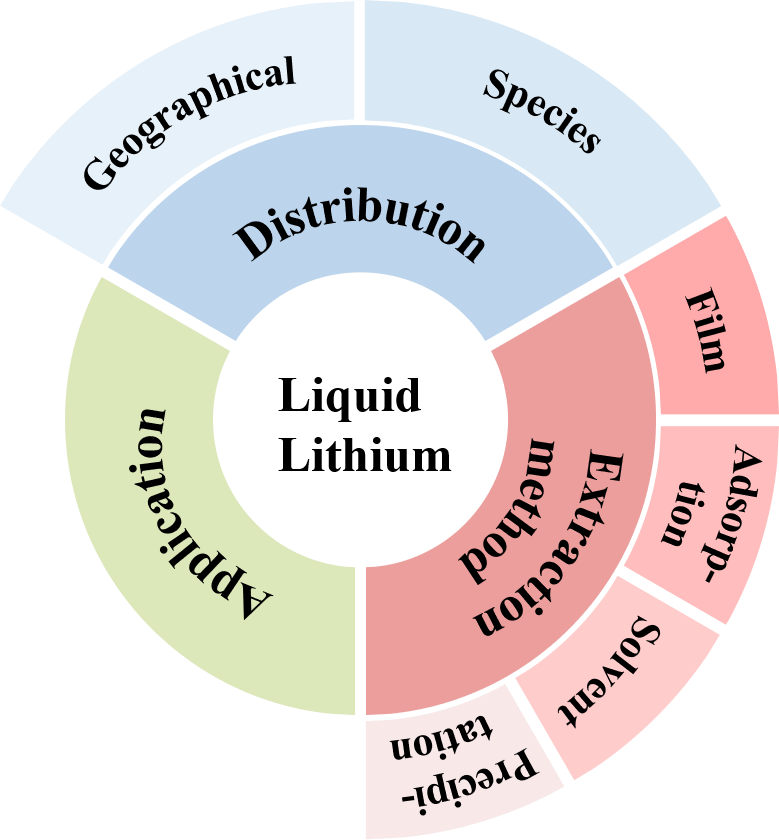
There has been a growing acknowledgment of the substantial importance of lithium as a pivotal mineral on a global scale. Prominent economies have strategically designated lithium as an essential mineral, underscoring its significance. However, despite the ample availability of lithium resources worldwide, their allocation is disparate, and demand is concentrated. Currently, liquid lithium resources serve as one of the primary sources in the mining industry, albeit with considerable challenges in extracting substantial quantities due to the scarcity of high-quality salt lake resources. This article aims to offer a comprehensive review of the present application and distribution status of lithium resources, with a specific focus on four principal techniques for extracting liquid lithium and the formation methods employed for lithium-ion sieve adsorbents. Additionally, we provide a comprehensive overview of recent advancements in diverse methodologies pertaining to the liquid lithium extraction. The principal aim of this review is to elucidate the current state of liquid lithium extraction, scrutinize and predict future developmental patterns, and ultimately furnish technical resources for both domestic and international stakeholders involved in the extraction of liquid lithium resources.
Contents
1 Introduction
1.1 Application of lithium
1.2 Distribution of lithium resources
2 Extraction technology of liquid lithium resources
2.1 Precipitate
2.2 Solvent extraction
2.3 Adsorption
2.4 Membrane separation process
3 Molding of lithium-ion sieve
3.1 Granulation
3.2 Magnetic material
3.3 Nanofiber and membrane
4 Conclusion and outlook
Zhennan Chen , Jingjing Du , Jianbo Shi . Extraction Technology of Liquid Lithium[J]. Progress in Chemistry, 2024 , 36(10) : 1559 -1566 . DOI: 10.7536/PC240217
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
USGS. Mineral Commodity Summaries 2023[R], 2023.
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
(黄维农, 孙之南, 王学魁, 乜贞, 卜令忠. 现代化工, 2008, (2): 14.)
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
(陈海霞, 严红, 孙云龙, 马国强. 无机盐工业, 2024, 56(9): 9.)
|
| [33] |
(张文丁. 中国科学院大学硕士论文, 2021.)
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
(唐珏, 王俊, 储瑶, 袁博, 崔祖霞. 矿产综合利用, 2023, (6):71.)
|
/
| 〈 |
|
〉 |