

Recent Development of Small Molecule Drugs for the Treatment of Osteoarthritis
Received date: 2024-11-07
Revised date: 2024-12-06
Online published: 2024-12-21
Osteoarthritis is a common degenerative disease in middle-aged and elderly people, and its lesions involve the entire joint, such as cartilage, subchondral bone and synovial membrane. Although there are some launched drugs that can be used to relieve the pain caused by osteoarthritis, the therapeutic effects are insufficient and there are some certain side effects. For now, except for artificial joint replacement for late stage of osteoarthritis, there are no effective drugs for early and middle stage of osteoarthritis to delay its progression. The treatment of osteoarthritis in clinical faces a huge demand for valid drugs, but the development of therapeutic drugs is way behind schedule. This review focuses on the progress of small molecule drugs for the treatment of osteoarthritis, especially the small molecule chemical entities which have entered the clinical trial stage. We hope this article will provide inspiration for the researchers on the drug development of osteoarthritis.
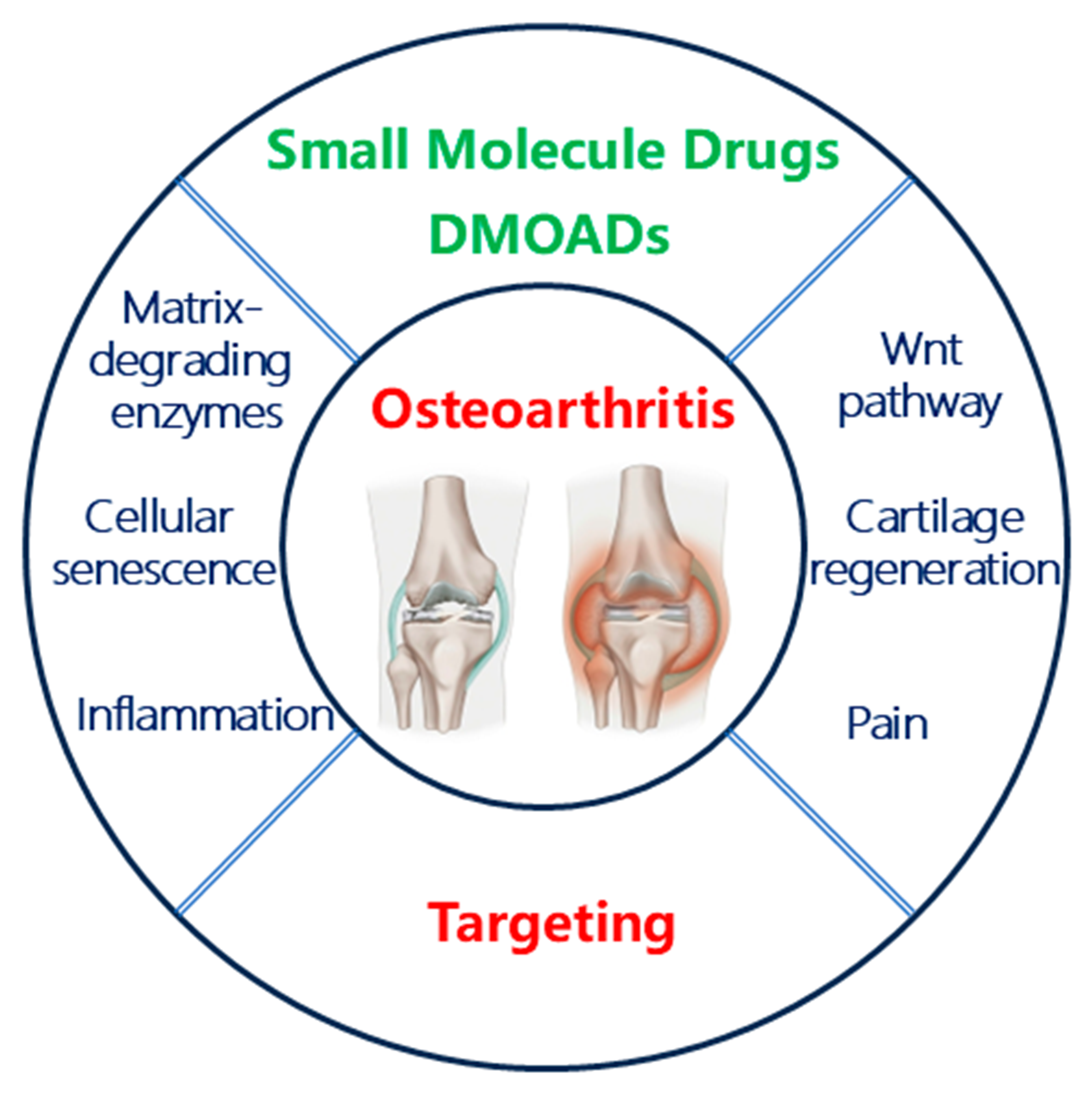
1 Introduction of osteoarthritis
2 Features of osteoarthritis
3 Small molecule drugs against osteoarthritis
3.1 Clinical small molecule drugs against osteoarthritis
3.2 The development of small molecule disease- modifying osteoarthritis drugs (DMOADs)
4 Conclusion and outlook
Key words: osteoarthritis; small molecule drugs; drug development
Xiaofei Zhang , Chunhao Yang . Recent Development of Small Molecule Drugs for the Treatment of Osteoarthritis[J]. Progress in Chemistry, 2024 , 36(12) : 1849 -1858 . DOI: 10.7536/PC241115
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
谢毓元, 陈文致. 化学通报, 1980, 5: 43.
|
| [73] |
谢毓元. 国外医学药学分册, 1984, 43.
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
/
| 〈 |
|
〉 |