

Green Chemical Technology for the Synthesis of Basic Organic Chemicals
Received date: 2024-11-13
Revised date: 2024-11-20
Online published: 2024-12-20
Supported by
National Natural Science Foundation of China(22072182)
The rapid development of the chemical industry has contributed to global economic growth and improved people's quality of life. However, the massive consumption of fossil fuels in the chemical production process has also caused environmental pollution and climate change that cannot be ignored. Academician Min Enze has been committed to directional fundamental research in green chemistry since 1990. He has introduced and integrated new materials, processes, and reaction pathways into the traditional petrochemical industry, led and guided the implementation of multiple major national basic research projects, accumulated new technological knowledge in green chemistry, laid the foundation for green refining and petrochemical new processes, and developed multiple influential green chemical technologies, effectively promoting the development of green technologies in the petrochemical industry. This article summarizes the green chemical technology practice cases formed on the basis of the catalytic new materials and green chemistry new knowledge laid by Academician Min Enze in the past 20 years, including the green production technology of caprolactam, fluidized bed hydrogen peroxide technology, complete technology of epoxy propane production by hydrogen peroxide method, and green synthesis technology of cyclohexene esterification and hydrogenation. These condensed innovative practices and experiences in catalytic materials and chemical engineering will provide a foundation and reference for us to carry out new technological innovations.
Xiaoxin Zhang . Green Chemical Technology for the Synthesis of Basic Organic Chemicals[J]. Progress in Chemistry, 2024 , 36(12) : 1859 -1873 . DOI: 10.7536/PC241116
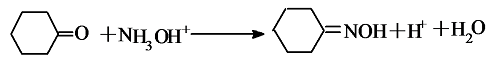
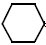 =O+ NH3 + H2O2 $\xrightarrow{\mathrm{TS}-1分子筛}$
=O+ NH3 + H2O2 $\xrightarrow{\mathrm{TS}-1分子筛}$ = NOH + 2H2O + Q
= NOH + 2H2O + Q| [1] |
(张晓昕. 催化学报, 2013, 34(1): 4.)
|
| [2] |
(何鸣元. 催化学报, 2013, 34(1): 10.)
|
| [3] |
(闵恩泽, 李成岳. 绿色石化技术的科学与工程基础. 北京: 中国石化出版社, 2002.)
|
| [4] |
(宗保宁, 潘智勇. 己内酰胺绿色生产技术的化学和工程基础. 北京: 科学出版社, 2020)
|
| [5] |
|
| [6] |
(何鸣元. 石油炼制和基本有机化学品合成的绿色化学. 北京: 中国石化出版社, 2005. 320.)
|
| [7] |
(夏长久, 杨焯, 林民, 朱斌, 彭欣欣, 汪燮卿, 舒兴田. 石油炼制与化工, 2024, 55(1): 18)
|
| [8] |
|
| [9] |
(孙斌, 程时标, 孟祥堃, 杨克勇, 吴巍, 宗保宁. 中国科学: 化学, 2014, 44(1): 40.)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
(潘智勇, 高国华, 杨克勇, 宗保宁. 中国科学: 化学, 2015, 45(5): 541)
|
| [16] |
|
| [17] |
|
| [18] |
(邓明杨, 张晓昕, 宗保宁. 中国科学: 化学, 2021, 51(7): 896)
|
| [19] |
(寇建朝, 张晓昕, 宗保宁. 化工进展, 2002, 21: 741)
|
| [20] |
(窦悦珊, 吕晓东. 当代石油石化, 2023, 31(5): 21)
|
| [21] |
|
| [22] |
(夏长久, 于佳元, 林民, 朱斌, 彭欣欣, 舒兴田. 石油炼制与化工, 2024, 55(1): 131)
|
| [23] |
|
| [24] |
|
/
| 〈 |
|
〉 |