

Research on Construction of Three-Dimensional Current Collector for Stabling Lithium Metal Anodes
Received date: 2024-03-11
Revised date: 2024-05-05
Online published: 2024-06-30
Supported by
National Natural Science Foundation of China(22209065)
Natural Science Foundation of Shandong Province(ZR2020MB082)
Natural Science Foundation of Shandong Province(ZR2021QE039)
Natural Science Foundation of Shandong Province(ZR2021QE149)
College Student Innovation and Entrepreneurship Project of Linyi University(X202310452295)
Lithium metal is considered to be the most promising anode material owing to its extraordinary theoretical specific capacity and the lowest redox potential. However, lithium anodes suffer from many challenges, such as the uncontrolled growth of lithium dendrites, unstable solid electrolyte interface (SEI) layers, and infinite volume expansion of lithium during cycling, which hinder the further commercial application of lithium metal batteries. Numerous important strategies have been proposed to overcome these challenges. Among them, three-dimensional current collectors can not only reduce the local current density and alleviate dendrite growth, but also mitigate the volume change of Li metal during the stripping/plating process. Based on the above problems, this review summarizes the working mechanisms and the latest research progress about the design of the three-dimensional structure and the lithiophilic modification to stabilize the lithium metal anode.
1 Introduction
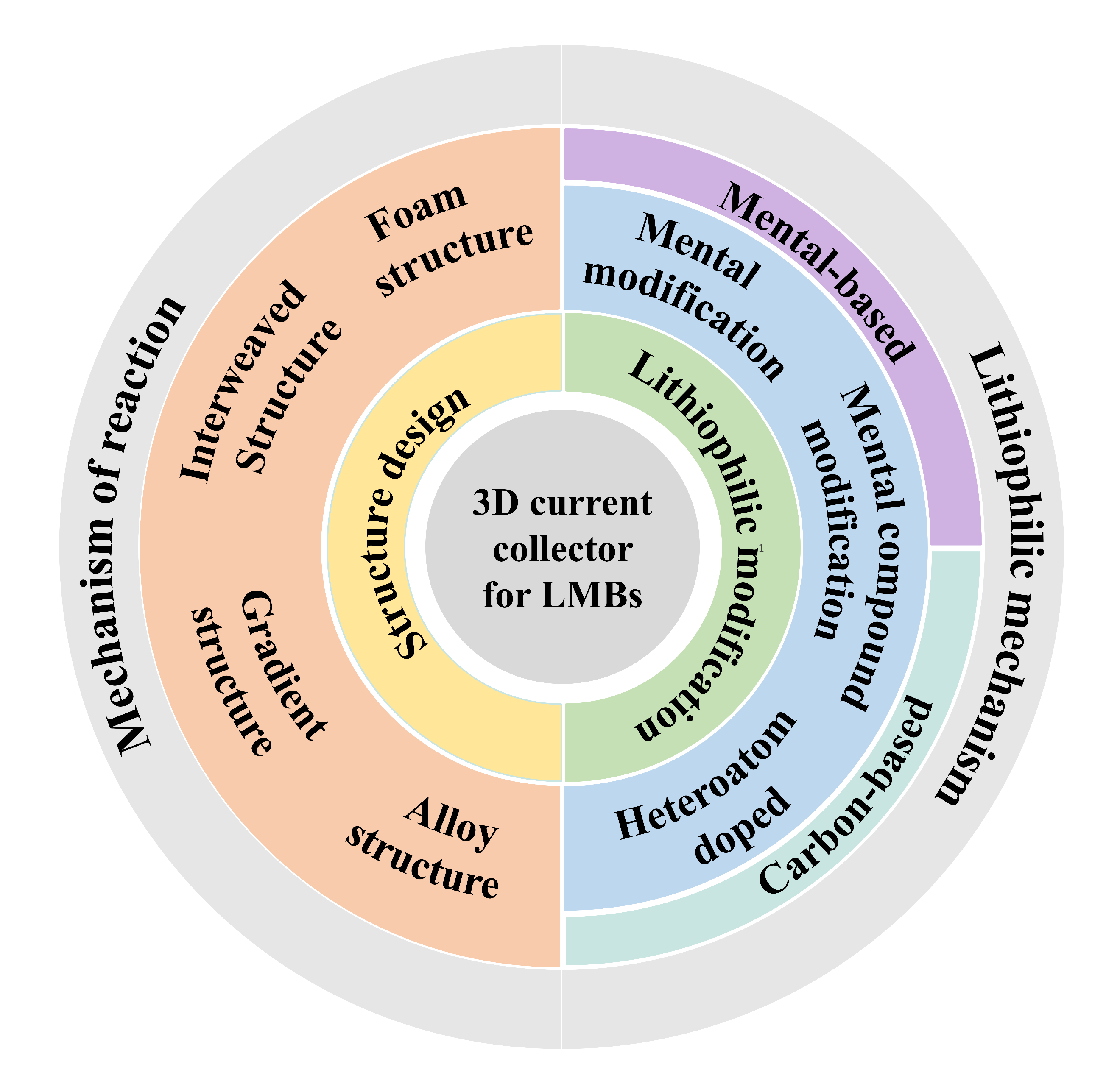
2 The design of three-dimensional current collector
2.1 Mechanism of action
2.2 Construction methods
2.3 Structural design
3 Lithiophilic modification
3.1 Lithiophilic mechanism
3.2 Surface modification methods
3.3 Metal-based current collectors
3.4 Carbon-based current collectors
4 Conclusion and outlook
Chenyang Li , Li Su , Qinglei Wang , Xuehui Shangguan , Lijun Gao , Faqiang Li . Research on Construction of Three-Dimensional Current Collector for Stabling Lithium Metal Anodes[J]. Progress in Chemistry, 2024 , 36(12) : 1929 -1943 . DOI: 10.7536/PC240317
图2 (a) 二维平面铜和三维多孔铜上锂沉积的结构变化示意图[20];(b) 平面和多孔铜箔上的电场以及相应的锂沉积位点示意图[21];(c) 平面铜箔和铜纳米线膜之间的锂离子通量分布和锂沉积模型的明显差异概要[22]Fig. 2 (a) Schematic of structural changes in depositing Li on 2D planar Cu and 3D porous Cu[20]. (b) Schematic of the electric field on planar and porous copper foils and the corresponding Li deposition sites[21]. (c) Synopsis of obvious difference of Li-ion flux distribution and Li plating models between planar Cu foil and Cu nanowire membrane[22] |
表1 四种三维集流体合成方法的优点和缺点Table 1 Advantages and disadvantages of four synthesis methods of the three-dimensional current collector |
| Methods | Advantages | Disadvantages |
|---|---|---|
| Electrospinning | Controllable and tunable fiber features; good scalability; simple operation; low cost | Highly corrosive or toxic solvents used for preparing the spin solution; difficulty in recycling; causing environmental pollution easily |
| Templating | Controllable fiber features | Complex operation process; high cost, time-consuming, difficulty in removing templates |
| Dealloying | Simple operation; low cost; easy mass production | Uncontrollable porous nanometal materials features |
| High-temperature carbonization | Simple operation; low cost | making the material brittle |
图5 (a) M-CuO@Cu电极上锂沉积模式的示意图[38];(b) GSZO-CF基材上的锂电镀工艺示意图[39];(c) GSZO-CF对称电池的电化学性能:50 mA·cm−2 电流密度,10 mAh·cm−2 锂固定沉积量下[39];(d) CBG合成过程示意图[40];(e) CNIP 纳米复合材料合成过程的机理示意图[41]Fig. 5 (a) Schematic illustration of the Li deposition patterns on M-CuO@Cu[38]. (b) Schematic illustration of Li plating process on GSZO-CF[39]. c)Electrochemical performance of symmetric cells[39] at 50 mA·cm−2 with a fixed Li deposition of 10 mAh·cm−2. (d) Schematic illustration of the synthesis procedure of CBG[40]. (e) Mechanism schematic of the CNIP nanocomposite synthesis process[41] |
图7 (a) 元素周期表中各种元素的电负性以及与锂反应的元素或化合物的吉布斯自由能变[46];(b) 铜箔和金基底上锂镀层的成核过电势[47];(c) 各种基底上锂成核过电势的比较[47]Fig. 7 (a) Electronegativities of various elements in the periodic table and ΔrG of elements or compounds reacted with the molten Li[46]. (b) Nucleation overpotential of Li plating on Cu foil and Au substrate[47]. (c) Comparison of Li nucleation overpotential on various substrates[47] |
表2 利用亲锂金属纳米结构修饰泡沫铜和泡沫镍的方法和性能参数Table 2 Methods and performance parameters of modifying copper foam by lithiophilic metal nanostructures |
| substrate | Lithiophilic materials | Cycling condition | Cycle performance/(h) | Overpotential/(mV) | Ref |
|---|---|---|---|---|---|
| Cu foam | ZnO Nanoflakes | (1mA·cm−2, 1mAh·cm−2) | 4000 | 13 | 51 |
| 3D Li2O@Cu nanowires array | (1mA·cm−2, 1mAh·cm−2) | 600 | 15 | 52 | |
| Li2O@Cu nanowires array | (1mA·cm−2, 1mAh·cm−2) | 500 | 10 | 53 | |
| Cu2S NWs | (1mA·cm−2, 1mAh·cm−2) | 150 | 50 | 54 | |
| Cu6Sn5@Cu2+1O nanowires | (1mA·cm−2, 1mAh·cm−2) | 1250 | 8 | 55 | |
| Ni foam | ZnO nanorods | (1mA·cm−2, 2mAh·cm−2) | 1200 | 12 | 57 |
| NiFx nanosheets | (1mA·cm−2, 1mAh·cm−2) | 1300 | 20 | 58 | |
| V2O5 nanobelt arrays | (1mA·cm−2, 1mAh·cm−2) | 1600 | 18 | 59 | |
| Hierarchical Oα-rich Co3O4 nanoarray | (1mA·cm−2, 1mAh·cm−2) | 800 | 32 | 60 | |
| Lithiated NiCo2O4 Nanorods | (1mA·cm−2, 1mAh·cm−2) | 1000 | 16 | 61 | |
| Ni3S2 layer | (1mA·cm−2, 1mAh·cm−2) | 1000 | 25 | 62 |
图9 (a) 杂原子掺杂碳和锂原子之间的计算结合能总结[64];(b) 锂在铜箔、石墨烯和N掺杂石墨烯电极上成核过电势[65];(c) 具有吡啶氮(pnN)、吡咯氮(prN)、边缘季氮(qN)和体相季氮(qnN)的N掺杂石墨烯的示意图[65];(d) 锂原子与Cu、石墨烯以及N掺杂石墨烯的不同官能团的结合能[65];(e) Cu、石墨烯和吡咯-N基团的锂原子吸附位点处的形变电荷密度[65]Fig. 9 (a) Summary of calculated binding energy between heteroatom-doped carbon and a Li atom[64]. (b) Nucleation overpotential on Cu foil, graphene (G), and NG electrodes[65]. (c) Schematic diagram of N-doped graphene with pyridinic nitrogen (pnN), pyrrolic nitrogen (prN), quaternary nitrogen on the edge (qN) and quaternary nitrogen in the bulk phase (qnN)[65]. (d) Binding energy of a Li atom with Cu, G, and different functional groups of N-doped graphene[65]. (e) The deformation charge density at a Li atom adsorption site of: Cu, graphene, and pyrrolic-N group[65] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
(程新兵, 张强. 化学进展, 2018, 30(1): 51.)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
(陈龙, 黄少博, 邱景义, 张浩, 曹高萍. 化学进展, 2021, 33(8): 1378.)
|
| [15] |
|
| [16] |
|
| [17] |
(郭驰, 张望, 涂吉, 陈盛锐, 梁济元, 郭向可. 化学进展, 2022, 34(2): 370.)
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
/
| 〈 |
|
〉 |