

Modification and Application of Bi2MoO6 in Photocatalytic Technology
Received date: 2022-09-29
Revised date: 2023-01-30
Online published: 2023-02-20
Supported by
development of Science and Technology of Jilin Province(YDZJ202201ZYTS629)
Natural Science Foundation Project of Jilin Province(YDZJ202201ZYTS356)
Natural Science Foundation Project of Jilin Province(YDZJ202101ZYTS073)
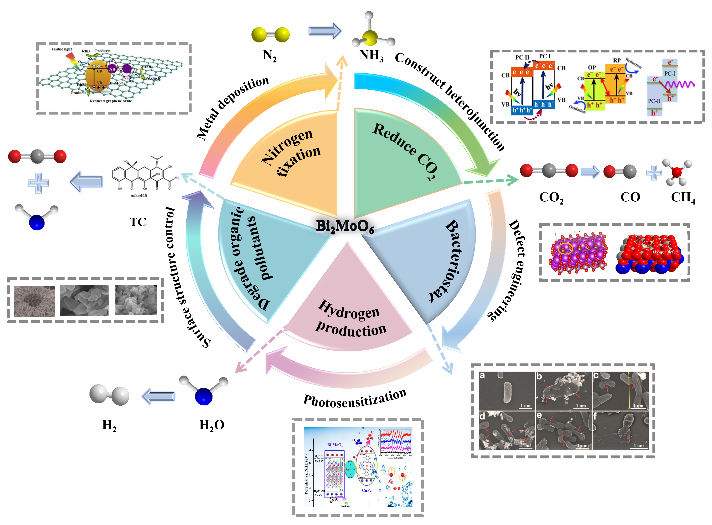
At present, ecological pollution and energy shortage have become global problems threatening human survival. Green and low energy consumption photocatalytic technology is of strategic significance to solve environmental disaster and energy crisis. As a ternary Aurivillius compound, Bi2MoO6 has attracted extensive attention of researchers due to its unique layered structure. However, the high carrier recombination rate limits its application in photocatalysis. This paper summarizes the strategies for modifying the performance of Bi2MoO6 based photocatalysts, such as surface structure tuning, defect engineering, metal deposition, heterojunction fabrication and photosensitization treatment. In many modification strategies, the influence of the construction of Bi2MoO6 based heterojunction on the photocatalytic performance has been mainly discussed. Finally, the current challenges faced by Bi2MoO6 based photocatalyst in photocatalysis technology are summarized, and the future development prospects are given, providing new ideas for accelerating the development of Bi2MoO6 based photocatalyst.
Dandan Wang , Zhaoxin Lin , Huijie Gu , Yunhui Li , Hongji Li , Jing Shao . Modification and Application of Bi2MoO6 in Photocatalytic Technology[J]. Progress in Chemistry, 2023 , 35(4) : 606 -619 . DOI: 10.7536/PC220934
表1 不同Bi2MoO6制备方法的对比Table 1 Comparison of different preparation methods of Bi2MoO6 |
| Photocatalyst | Degraded substance | Degradation efficiency | Preparation method | Advantages | Disadvantages | ref |
|---|---|---|---|---|---|---|
| Bi2MoO6 | RhB 5 mg·L-1 | 85% | Hydrothermal | Purity | High temperature and pressure | 26 |
| Bi2MoO6 | 4-CP | 91.64% | Sol-gel | Uniformity | Poor sintering | 27 |
| 8h-Bi2MoO6 | MO 10 mg·L-1 | 100% | In situ synthesis | Small particle | Uncontrollability | 28 |
| Bi2MoO6 | RhB 5 mg·L-1 | 99% | Microwave | Stability | Large gap | 29 |
| γ-Bi2MoO6 | RhB 10 mg·L-1 | 97.48% | Coprecipitation | Simple process | Reunite | 30 |
表2 近年来已报道的各类改性Bi2MoO6基光催化剂降解有机污染物性能的比较Table 2 Comparison of various modified Bi2MoO6 based photocatalysts reported for organic pollutant degradation in recent years |
| Photocatalyst | Organic Pollutants | Degradation efficiency | Modification method | Light conditions | ref |
|---|---|---|---|---|---|
| 2%Pd-Bi2MoO6(100 mg) | Phenol(5 mg·L-1,100 mL) | 100%(300 min) | Doping Pd | 300 W halogen lamp, λ≥ 410 nm | 49 |
| 0.01B-BMO(200 mg) | RhB(5 mg·L-1,100 mL) | 89%(50 min) | Doping B | 250 W halogen lamp, λ≥ 420 nm | 40 |
| Ag-Bi2MoO6(200 mg) | RhB(1×10-5 M,100 mL) | 98%(150 min) | Loaded Ag | Xe lamp | 50 |
| Bi2MoO6(50 mg) | RhB(10 mg·L-1,50 mL) | 96%(60 min) | Built piezoelectric polarization | 300 W Xe lamp, λ≥ 420 nm | 51 |
| vis/Bi2MoO6/PMS/Fe3+ | ATZ(2.5 mg·L-1,150 mL) | 99%(20 min) | Addition of Fe3+ | 300 W Xe lamp, λ≥ 415 nm | 52 |
| PAN/Bi2MoO6/Ti3C2 (20 mg) | TC(15 mg·L-1,100 mL) | 90.3%(30 min) | Fiber membrane adsorption | 300 W Xe lamp, λ≥ 420 nm | 53 |
表3 近年来已报道的各类改性Bi2MoO6基光催化剂还原CO2性能的比较Table 3 Comparison of various modified Bi2MoO6 based photocatalysts reported for CO2 reduction in recent years |
| Photocatalyst | Production | Production rate | Modification method | Light | ref |
|---|---|---|---|---|---|
| Au1.5/HMS-BMO(100 mg) | CH4 | 37.6 μmol·g-1·h-1 | Au NPs supported | 300 W Xe lamp λ≥ 420 nm | 54 |
| Bi2@Ti1 | CH3OH | 27.1 μmol·g-1·h-1 | Oxygen deficient | UV-visible light | 55 |
| RP-BMO | C2H5OH | 51.81 μmol·g-1·h-1 | RP decorated | Xe lamp λ≥ 400 nm | 56 |
| Bi2MoO6/rGO(20 mg) | CH3OH C2H5OH | 21.2 μmol·g-1·h-1 14.38 μmol·g-1·h-1 | BM QDs | visible Light | 57 |
| BM-HFMS(50 mg) | CH3OH C2H5OH | 6.2 μmol·g-1·h-1 4.7 μmol·g-1·h-1 | Hierarchical flower-like | 300 W Xe lamp λ≥ 420 nm | 58 |
| Bi2MoO6/MnP(10 mg) | CH3OH | 11.88 μmol·g-1·h-1 | Organic-inorganic | 500 W Xe lamp λ≥ 420 nm | 59 |
| Ov-Bi2MoO6(50 mg) | CH3OH C2H5OH | 35.5 μmol·g-1·h-1 3.43 μmol·g-1·h-1 | Oxygen vacancy and 3D structure | 300 W Xe lamp λ≥ 420 nm | 60 |
| BMO-U-P(10 mg) | CO | 14.38 μmol·g-1·h-1 | CTAB-assisted and corona | visible light | 61 |
表4 近年来已报道的各类Bi2MoO6基异质结光催化剂降解性能的比较Table 4 Comparison of Bi2MoO6 based heterojunction photocatalysts reported for organic pollutant degradation in recent years |
| Photocatalyst | Organic Pollutants | Degradation efficiency | Heterojunction type | Light conditions | ref |
|---|---|---|---|---|---|
| Bi2MoO6/g-C3N4(10 mg) | MB(20 mg·L-1,50 mL) | 92.71%(150 min) | 2D/2D Z-scheme | Visible light irradiation | 77 |
| AgI/Ag/Bi2MoO6(100 mg) | RhB(20 mg·L-1,100 mL) | 93.6%(15 min) | Z-scheme | 400 W Xe lamp, λ≥ 400nm | 78 |
| Ag3PO4/RGO/Bi2MoO6(25 mg) | MB(20 mg·L-1,50 mL) | 97.53%(25 min) | Z-scheme | 65W visible lamp | 79 |
| CF/C3N4/Bi2MoO6 | TC (20 mg·L-1,100 mL) Cr(Ⅵ)(50mg·L-1, 100 mL) | 86%(60 min) 80%(60 min) | S-scheme | 300 W Xe lamp, λ≥ 420 nm | 80 |
| BMO/CN-2(30 mg) | TC(20 mg·L-1,100 mL) Cr(Ⅵ)(10 mg·L-1, 100 mL) | 88.1%(60 min) 96.7%(60 min) | 0D/2D S-scheme | 300 W Xe lamp, λ≥ 420 nm | 81 |
| Bi2MoO6-Bi2O3-Ag3PO4 (30 mg) | TC(40 mg·L-1,30 mL) | 74.4%(120 min) | Ternary n-n-p | 400 W Xe lamp, λ≥ 420 nm | 82 |
表5 各类异质结的优缺点的比较Table 5 Comparison of advantages and disadvantages of various heterojunctions |
| Photocatalyst | Heterojunction type | Advantage | Disadvantage | Active species | ref |
|---|---|---|---|---|---|
| Bi2MoO6/BiVO4/g-C3N4 | Type-I | e--h+ | Redox ability; Converge | h+, e- | 89 |
| CdS-Bi2MoO6 | Type-II | e--h+ | Redox ability | h+, e- | 90 |
| H3PW12O40/TiO2-In2S3 | Type-Ⅲ | e--h+ | Reduction ability; broken gap | h+,·OH | 91 |
| Bi2MoO6/Bi12SiO20 | Z-Scheme | e--h+; Redox ability | Light shielding effect; media | h+,·O2- | 92 |
| g-C3N4/Bi2MoO6 | S-Scheme | e--h+; Redox ability; Internal electric field; Coulomb force; Band bending | Carriers separation efficiency | ·O2-,·OH | 93 |
| Fe3O4/N-Bi2MoO6 | p-n | e--h+; Space charge region | External magnetic field | h+,·O2- | 94 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
王杰. 上海工程技术大学硕士论文, 2019.).
|
| [16] |
|
| [17] |
赵依凡, 毛琦云, 翟晓雅, 张国英. 化学进展. 2021, 33(8): 1331. ).
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
夏宇. 深圳大学硕士论文, 2020.).
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
王敏, 杨长秀, 郑浩岩, 郭鹏瑶, 宋恩军. 硅酸盐学报. 2015, 43(11): 1643.).
|
| [27] |
|
| [28] |
张琴, 汪晓凤, 段芳, 陈明清. 无机化学学报, 2015, 31(11): 2152.).
|
| [29] |
张婷, 黄剑锋, 曹丽云, 周森. 硅酸盐学报. 2013, 41(5): 710.).
|
| [30] |
左广玲, 叶红勇, 李入林. 化工环保. 2015, 35(1): 89.).
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
/
| 〈 |
|
〉 |