

9,9'-Bianthracene-Based Blue Fluorescent Emitters for High-Performance Organic Light-Emitting Diodes
Received date: 2024-05-22
Revised date: 2024-08-07
Online published: 2024-09-18
Supported by
National Nature Science Foundation of China(22379105)
National Nature Science Foundation of China(62305202)
Natural Science Foundation of Shanxi Province(20210302123110)
Key Research and Development Program of Shaanxi(2023-YBGY-198)
Ningxia Sinostar Display Material Co., Ltd. Open Fund Project, Science and Technology Innovation Talent Team Project of Shanxi Province(202204051001013)
Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province(20230007)
Key Research and Development Program of Xianyang(L2023-ZDYF-QYCX-061)
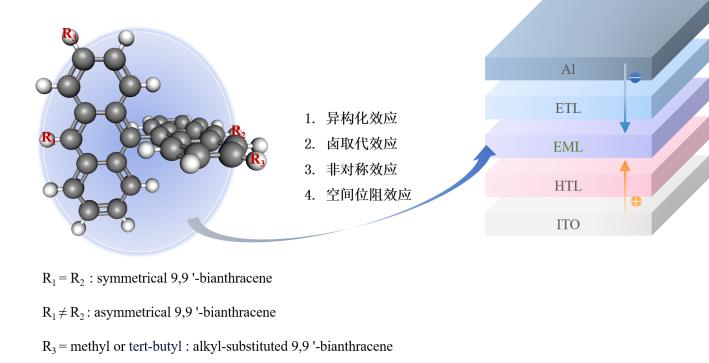
Organic light emitting diodes (OLEDs) have attracted extensive attention and research interest in advanced display and solid-state lighting due to their self-luminescence, low drive voltage, wide color gamut, surface luminescence, flexibility and rapid response. One of the primary colors of OLED, the development of blue emitter is still lagging far behind. Interestingly, 9,9'-bianthracene as a promising blue-emitter for high-performance fluorescent OLEDs exhibits excellent optoelectronic performance in recent years. Here, we review the progress with the development of 9,9'-anthracene-based blue fluorescent materials and gain insight into their contribution towards enhanced OLED performance. Different approaches to achieve blue emission from molecular design including isomerization, fluorine substitution, asymmetrical structuring, and steric hindrance effects are discussed, with particular focus on device efficiency and stability. Furthermore, an outlook for future challenges and opportunities of OLEDs from the development of new molecular structures, understanding of luminescence mechanisms as well as innovation in flexible and large-scale panels is provided.
1 Introduction
1.1 OLED structure and principle
1.2 OLED emissive materials
2 9,9'-Bianthracene-based blue light-emitting materials and device performance
2. 1 Basic structure of bianthracene
2. 2 9,9'-Bianthracene-based blue light-emitting materials and devices
2. 3 Structures and chemical properties of other bianthracene derivatives
2. 4 BT. 2020 blue light
Correlation between the structure and performance of 9,9'-Bianthracene-based blue light-emitting materials
3. 1 Isomerization effects in bianthracene
3. 2 Halogen substitution effects
3. 3 Asymmetric effects
3. 4 Steric hindrance effects
3. 5 Blue-emitting device design
4 Conclusion and prospects
4. 1 Summary
4. 2 Prospects
Aowei Zhu , Zhanfeng Li , Kunping Guo , Yanqin Miao , Baoyou Liu , Gang Yue . 9,9'-Bianthracene-Based Blue Fluorescent Emitters for High-Performance Organic Light-Emitting Diodes[J]. Progress in Chemistry, 2025 , 37(3) : 317 -331 . DOI: 10.7536/PC240520
图2 Schematic Diagram of Classification and Luminescence Mechanism of Blue OLED MaterialsFig. 2 Schematic diagram of the emission mechanism of different types of blue OLED materials |
图5 Schematic Diagram of Molecular Structure of Symmetric 9,9'-Bianthracene-Based Blue Light MaterialsFig. 5 Schematic diagram of the molecular structure of symmetric 9,9'-bianthracene blue light-emitting materials |
表1 对称9,9'-联蒽类蓝光材料的OLED器件性能总结Table 1 Performance summary of OLED devices using symmetrical 9,9'-bianthracene blue light materials |
| Compound | EL λ (nm) | CE (cd·A-1) | PE (lm·W-1) | L max (cd·m-2) | EQEmax (%) | CIE (x, y) | Ref |
|---|---|---|---|---|---|---|---|
| (1)a | 472 | 3.95 | 3.87 | 6470 | 2.47 | (0.17, 0.22) | 48 |
| (1)b | 432 | 1.36 | 0.85 | 1860 | 3.46 | (0.16, 0.05) | 48 |
| (1)c | 472 | 12.06 | 5.59 | 19 200 | 5.72 | (0.15, 0.33) | 48 |
| (2)a | 446 | 2.11 | 1.74 | 788 | 1.9 | (0.16, 0.12) | 54 |
| (2)c | 459 | 5.18 | 3.72 | 2059 | 3.52 | (0.15, 0.19) | 54 |
| (5)a | 444 | 1.87 | 0.92 | 1383 | 1.1 | (0.17, 0.16) | 56 |
| (5)c | 468 | 6 | 2.88 | 8726 | 3.19 | (0.16, 0.27) | 56 |
| (6)a | 450 | 2.21 | 1.51 | 1881 | 2.02 | (0.16, 0.12) | 57 |
| (7)a | 460 | 3.58 | 2.58 | 2367 | 1.94 | (0.26, 0.26) | 57 |
| (8)a | 450 | 4.27 | 2 | — | 4.26 | (0.15, 0.12) | 58 |
| (9)a | 499 | 2.72 | 1.14 | — | 1.5 | (0.21, 0.29) | 58 |
| (10)b | 438 | 1.66 | 1.02 | 1647 | 2.67 | (0.15, 0.08) | 60 |
| (10)c | 472 | 8.8 | 5.79 | 25 065 | 4.14 | (0.17, 0.33) | 60 |
| (11)b | 440 | 1.28 | 0.89 | 1669 | 1.99 | (0.15, 0.08) | 60 |
| (11)c | 468 | 8.67 | 4.93 | 25 832 | 4.86 | (0.16, 0.27) | 60 |
| (12)b | 437 | 1.22 | 0.87 | 2766 | 1.86 | (0.15, 0.08) | 60 |
| (12)c | 468 | 9.64 | 6.77 | 28 667 | 5.43 | (0.15, 0.27) | 60 |
| (13)b | 435 | 1.47 | 1.1 | 2016 | 2.75 | (0.15, 0.07) | 60 |
| (13)c | 468 | 7.05 | 4.46 | 23 887 | 3.35 | (0.18, 0.37) | 60 |
| (14)b | 437 | 2.11 | 1.45 | 838 | 3.56 | (0.15, 0.08) | 60 |
| (14)c | 478 | 7.98 | 5.65 | 17 452 | 4.02 | (0.17, 0.31) | 60 |
| (15)a | 444 | 1.11 | 0.46 | 2136 | 0.93 | (0.15, 0.13) | 62 |
| (15)c | 468 | 5.88 | 2.69 | 12 690 | 2.98 | (0.15, 0.30) | 62 |
| (16)a | 444 | 0.94 | 0.36 | 1919 | 0.84 | (0.16, 0.17) | 61 |
| (16)c | 472 | 4.38 | 0.36 | 14 200 | 2.29 | (0.16, 0.32) | 61 |
| (17)b | 435 | 3.05 | 2.62 | 3588 | 5.02 | (0.15, 0.08) | 59 |
| (18)a | — | 0.63 | 0.42 | 2290 | 0.31 | (0.21, 0.29) | 63 |
| (19)a | 444 | 2.94 | 1.9 | 3112 | 2.22 | (0.18, 0.17) | 57 |
| (20)a | — | 2.24 | 1.78 | 15 600 | 0.97 | (0.28, 0.33) | 63 |
| (21)a | 448 | 3.7 | 3.9 | 6137 | 3.9 | (0.15, 0.10) | 64 |
a Undoped device.b Devices prepared by doping as guest materials.c Devices prepared by doping as host materials. |
图6 Molecular Structure Diagram of Asymmetric 9,9'-Bianthracene Blue Light MaterialsFig. 6 Schematic diagram of the molecular structure of asymmetric 9,9'-bianthracene blue light-emitting materials |
表2 非对称9,9'-联蒽类蓝光材料的OLED器件性能总结Table 2 Performance summary of OLED devices using asymmetrical 9,9 '-bianthracene blue light materials |
| Compound | EL λ(nm) | CE (cd·A-1) | PE (lm·W-1) | L max (cd·m-2) | EQEma (%) | CIE (x, y) | Ref |
|---|---|---|---|---|---|---|---|
| (22)a | 455 | 5.1 | 2.33 | — | 4.02 | (0.17, 0.15) | 58 |
| (23)a | 501 | 7.79 | 3.79 | — | 3.17 | (0.19, 0.52) | 58 |
| (24)a | 466 | 6.78 | 3.55 | — | 4.71 | (0.15, 0.20) | 58 |
| (25)a | 466 | 8.97 | 4.43 | — | 6.37 | (0.14, 0.19) | 58 |
| (26)a | 469 | 2.1 | 1.16 | 959 | 1.74 | (0.16, 0.22) | 65 |
| (27)a | 481 | 2.7 | 1.89 | 935 | 1.55 | (0.18, 0.24) | 65 |
| (28)a | 464 | 4.85 | 3.83 | 7175 | 2.72 | (0.17, 0.22) | 67 |
| (28)c | 512 | 21.39 | 15.9 | 74 922 | 6.69 | (0.28, 0.63) | 67 |
| (29)a | 452 | 5.93 | 4.91 | 10 608 | 4.09 | (0.16, 0.16) | 67 |
| (29)c | 508 | 23.83 | 19.19 | 82 235 | 7.13 | (0.27, 0.61) | 67 |
a Undoped device.b Devices prepared by doping as guest materials.c Devices prepared by doping as host materials. |
图7 Molecular Structure Diagram of Alkyl-Substituted 9,9'-Bianthracene Blue Light MaterialsFig. 7 Schematic diagram of the molecular structure of alkyl- substituted 9,9'-bianthracene blue light-emitting materials |
表3 烷基取代9,9'-联蒽类蓝光材料的OLED器件性能总结Table 3 Summary of OLED devices using alkyl-substituted 9,9 '-bianthracene blue light-emitting materials |
| Compound | EL λ (nm) | CE (cd·A-1) | PE (lm·W-1) | L max (cd·m-2) | EQEmax (%) | CIE (x, y) | Ref |
|---|---|---|---|---|---|---|---|
| (30)a | 456 | 2.26 | 1.92 | 3546 | 2.51 | (0.15, 0.10) | 48 |
| (30)b | 436 | 2.76 | 2.4 | 3742 | 3.6 | (0.16, 0.08) | 48 |
| (30)c | 468 | 16.54 | 16.57 | 13 800 | 9.47 | (0.15, 0.26) | 48 |
| (31)a | 440 | 2.52 | 2.73 | 2013 | 3.2 | (0.15, 0.06) | 48 |
| (31)b | 436 | 4.16 | 3.84 | 2310 | 4.56 | (0.15, 0.07) | 48 |
| (31)c | 460 | 11.33 | 10.45 | 10 900 | 7.16 | (0.15, 0.20) | 48 |
| (32)a | 444 | 2.74 | 2.26 | 965 | 3.94 | (0.16, 0.07) | 57 |
| (32)c | 460 | 9.97 | 8.73 | 6822 | 6.96 | (0.14, 0.18) | 57 |
| (33)a | 436 | 2.07 | 1 | 2284 | 2.43 | (0.17, 0.09) | 68 |
| (33)b | 440 | 2.46 | 1.45 | 3860 | 4.57 | (0.15, 0.05) | 68 |
| (33)c | 480 | 20.7 | 46.24 | 23 670 | 8.69 | (0.17, 0.40) | 68 |
| (34)a | 444 | 2.35 | 1.73 | 3450 | 1.81 | (0.18, 0.15) | 68 |
| (34)b | 444 | 2.93 | 1.96 | 5489 | 4.11 | (0.15, 0.07) | 68 |
| (34)c | 480 | 19.24 | 12.42 | 20 920 | 8.42 | (0.16, 0.38) | 68 |
| (35)a | 440 | 4.63 | 4.15 | 1110 | 6.11 | (0.15, 0.05) | 68 |
| (35)b | 444 | 4.09 | 3.67 | 3570 | 6.67 | (0.15, 0.05) | 68 |
| (35)c | 476 | 23.01 | 23.99 | 21 190 | 11.52 | (0.16, 0.38) | 68 |
| (36)a | 444 | 1.18 | 0.57 | 1423 | 1.66 | (0.15, 0.07) | 68 |
| (36)b | 440 | 4.43 | 4.21 | 3316 | 6.25 | (0.15, 0.05) | 68 |
| (36)c | 476 | 18.63 | 12.9 | 22 010 | 7.9 | (0.17, 0.40) | 68 |
| (37)a | 448 | 2.64 | 1.9 | 2584 | 3.61 | (0.14, 0.09) | 67 |
| (37)b | 444 | 2.58 | 2.23 | 2769 | 4.66 | (0.14,0.06) | 67 |
| (38)a | 444 | 2.45 | 1.62 | 2364 | 3.03 | (0.15, 0.10) | 67 |
| (38)b | 448 | 2.34 | 1.77 | 2978 | 3.85 | (0.14, 0.07) | 67 |
| (39)a | 452 | 2.44 | 1.08 | 1444 | 2.54 | (0.14, 0.15) | 67 |
| (39)b | 444 | 2.22 | 1.74 | 2775 | 4.16 | (0.14, 0.06) | 67 |
| (40)a | 448 | 2.78 | 1.94 | 248 | 3.72 | (0.14, 0.09) | 67 |
| (40)b | 440 | 2.85 | 2.49 | 663 | 5.25 | (0.14, 0.06) | 67 |
| (41)a | 448 | 4.77 | 3.37 | 217 | 3.07 | (0.15,0.21) | 67 |
| (41)b | 440 | 2.66 | 2.14 | 386 | 5.03 | (0.14, 0.05) | 67 |
| (42)a | 456 | 2.03 | 1.58 | 1164 | 2.66 | (0.14, 0.09) | 70 |
| (42)b | 444 | 0.95 | 0.66 | 3370 | 1.72 | (0.16, 0.07) | 70 |
| (42)c | 468 | 11.2 | 8.72 | 21 250 | 6.89 | (0.15, 0.25) | 70 |
| (43)a | 452 | 1.95 | 1.46 | 1458 | 3.16 | (0.15, 0.07) | 70 |
| (43)b | 448 | 0.92 | 0.73 | 3168 | 1.51 | (0.15, 0.07) | 70 |
| (43)c | 464 | 6.11 | 3.91 | 9256 | 4.11 | (0.15, 0.22) | 70 |
| (44)a | 448 | 1.04 | 0.45 | 1261 | 1.57 | (0.15, 0.08) | 70 |
| (44)b | 444 | 0.8 | 0.61 | 2744 | 1.1 | (0.16, 0.08 | 70 |
| (44)c | 464 | 7.35 | 4.2 | 9563 | 4.91 | (0.15, 0.22) | 70 |
| (45)a | 452 | 1.75 | 1.14 | 1138 | 2.78 | (0.15, 0.07) | 70 |
| (45)b | 452 | 1.07 | 0.83 | 2363 | 1.38 | (0.15, 0.09) | 70 |
| (45)c | 464 | 8.64 | 6.76 | 9913 | 5.88 | (0.15, 0.21) | 70 |
| (46)a | 444 | 0.63 | 0.3 | 963 | 1.04 | (0.15, 0.07) | 70 |
| (46)b | 452 | 1.04 | 0.77 | 2569 | 1.16 | (0.15, 0.11) | 70 |
| (46)c | 460 | 4.24 | 3.11 | 11 120 | 2.86 | (0.15, 0.21) | 70 |
a Undoped device.b Devices prepared by doping as guest materials.c Devices prepared by doping as host materials. |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
(董馨雨, 丁文超, 陈美玲. 中国照明电器, 2023(10): 40.).
|
| [6] |
(焦志强, 黄清雨, 张娟, 李晓虎, 袁广才. 新材料产业, 2018, (2): 25.).
|
| [7] |
(项兴焜. 电子测试, 2022(24): 139.).
|
| [8] |
(徐汀. 材料研究与应用, 2022, 16(5): 730.).
|
| [9] |
|
| [10] |
(邹建华, 朱冠成, 王磊, 徐苗, 吴为敬, 彭俊彪. 发光学报, 2023, 44(1): 198.).
|
| [11] |
(马永强. 机械管理开发, 2020, 35(11): 295.).
|
| [12] |
(孙阿辉, 李耀召, 陈果, 魏斌. 光学学报, 2022, 42(4): 171.).
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
(崔东岳, 王帅, 李淑红, 刘云龙, 王文军. 发光学报, 2021, 42(5): 691.).
|
| [18] |
|
| [19] |
(唐振宇, 郭浩清, 肖静, 陈志坚, 肖立新. 发光学报, 2023, 44(1): 26.).
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
(田禾, 苏建华. 世界科学, 1996, (11): 24.).
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
(娄敬丽, 黎刚刚, 王志明, 唐本忠. 发光学报, 2023, 44(1): 37.).
|
| [38] |
|
| [39] |
(张秀菊, 许运华, 史华红. 化学进展, 2006, 18(7/8): 870.).
|
| [40] |
(张慧东, 谷盼盼, 张芳, 都明旭, 叶开其, 刘宇. 高等学校化学学报, 2021, 42(12): 3571.).
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
(薛云娜, 柴生勇, 别国军, 刘波, 甘宁. 现代显示, 2008, (04): 29.).
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
(吕响. 太原理工大学硕士论文, 2017.).
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
/
| 〈 |
|
〉 |