

Morphological Evolution of Atmospheric Black Carbon Particles
Received date: 2024-05-11
Revised date: 2024-07-20
Online published: 2024-09-06
Supported by
National Natural Science Foundation of China(42107125)
National Natural Science Foundation of China(72104132)
Youth Foundation of MOE (Ministry of Education in China) Liberal arts and Social Sciences(21YJCZH136)
Shanxi Province Basic Research Program Foundation(20210302124201)
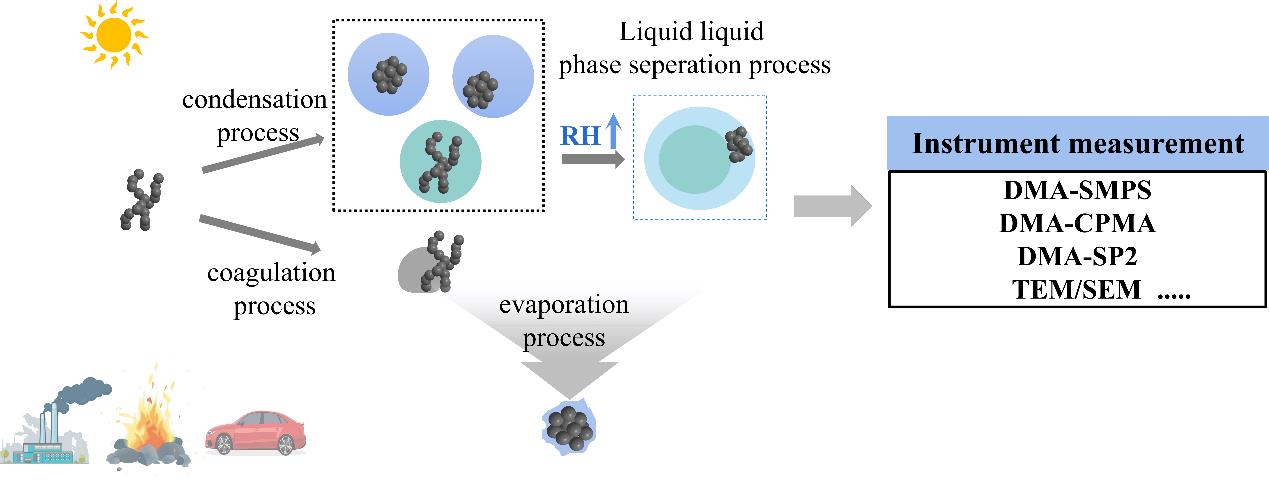
Black carbon (BC) particulate matter has significant light-absorbing capacity and is an important species contributing to haze pollution and global warming. However, quantitative studies of the light absorption capacity of black carbon (BC) have long been unable to reach a consensus affecting the accurate assessment of its environmental and climate effect. The morphological evolution of BC particles is the important factor affecting the light-absorbing capacity. However, the current literature review lacks a comprehensive summary of the characteristics and mechanisms involved in the evolution of BC micromorphology. This review summarizes the relevant studies on BC morphology evolution in recent years including the quantitative parameters of BC morphology, measurement and calculation methods of morphology parameters, the micromorphology evolution characteristics of BC during condensation process, phase separation process, coagulation process and evaporation process, and its evolution mechanism and main influencing factors. The evolution of the microphysical morphology of BC particles during different aging processes is the key to explaining the controversy over the light absorption of BC particles. However, there are still many uncertainties in the morphology evolution of BC core and the quantitative assessment of light absorption of complex-structured BC particles in these processes. Therefore, tracking the actual atmospheric BC morphology evolution, further investigating the effect of morphology evolution mechanism on the BC core collapse, and improving the models of BC light absorption and radiation will be the key research direction in the future.
1 Introduction
2 Quantitative characterization parameters and related measurement instruments for morphology of BC particles
2.1 Quantitative characterization parameters for morphology of BC particles
2.2 Related measurement instruments for morphology of BC particles
3 Morphological evolution characteristics and absorption effect of BC particles during different aging processes
3.1 Condensation process
3.2 Phase separation process
3.3 Coagulation process
3.4 Evaporation process
4 Conclusion and prospect
Kexin Liu , Zhuofei Du , Xin Gong , Hongjun Mao , Jianfei Peng . Morphological Evolution of Atmospheric Black Carbon Particles[J]. Progress in Chemistry, 2025 , 37(3) : 397 -410 . DOI: 10.7536/PC240510
表1 BC性质常见测量仪器Table 1 Common measuring instruments for BC properties. |
| Full name of the instrument | Abbreviation name of the instrument | Measurement principle | Main measurement parameters | Particle size range | Main advantages and disadvantages | online/offline |
|---|---|---|---|---|---|---|
| Single Particle soot photometer | SP2 | Laser induced incandescence | Mass of single particle BC, mix state | 70~700 nm | Quantitative analysis of single particle mix state; Limited measurement range of particle size | online |
| Soot Particle Aerosol Mass Spectrometry | SP-AMS | Laser induced incandescence, mass spectrum | Mass ratio of BC to non BC components | PM1.0 | Measure the chemical composition of BC; Only the mixed state of particle groups can be measured under conventional configuration | online |
| Aerosol Particle Mass Analyzer | APM | Balance of centrifugal force and electrostatic force | Mass of BC particles | 14 nm~1.3 μm | Fast and high-precision separation ability for measurement; Particle aggregation leads to reduced scanning efficiency and a limited range of measured particle sizes | online |
| Centri-fugal particle mass analyzer | CPMA | Balance of centrifugal force and electrostatic force | Mass of BC particles | 7 nm~1.3 μm | online | |
| Scanning Mobility Particle Spectrometer | SMPS | Different particle sizes have different electromigration rates | BC particle size and number concentration | 10~680 nm | Not dependent on the optical and fluid properties of particles; Low detection rate for particles below 200nm | online |
| Aerodynamic Aerosol Classifier | AAC | Aerodynamics | BC particle size | 25 nm~5 μm | No need to charge, using air jet technology to screen reduce the aggregation of small particle samples; Limited screening accuracy | online |
| Volatility tandem differential mobility analyzer | VTDMA | Changes in particle size before and after heating | Changes in particle size before and after volatilization | 100~600 nm | Direct measurement of the particle size changes before and after heating, without parameter assumptions; Extremely low volatile organic compounds may remain after heating | online |
| Humidified tandem differential mobility analyzer | HTDMA | Changes in particle size before and after humidification | Hygroscopic growth factor | 100~600 nm | Direct measurement of hygroscopic growth factor | online |
| | TEM | Laser irradiation generates secondary electrons in the sample | BC microstructure | Direct measurement of the surface morphology of BC; Laser high temperature causes evaporation of the coating material, Low scanning time resolution | offline | |
| Scanning Electron Microscope | SEM | Transmission electron imaging | offline |
图1 Schematic Diagram of BC Collapse Mechanism: The black circles in the figure represent carbon pellets, and the gray shadow represents the encapsulation layer: (a) Capillary Collapse Mechanism, (b) Droplet Activation Mechanism, (c) Evaporation Collapse Mechanism (The image is redrawn from Reference 88).Fig. 1 Schematic diagram of BC collapse mechanism, with black circles representing carbon balls and gray shadows representing the coating layer. (a) capillary collapse mechanism, (b) droplet activation mechanism, (c) evaporation collapse mechanism. (Images modified from ref 88) |
图3 (a) Redistribution Structure[67], (b) BC Adhesion to Other Aerosol Surfaces[86], (c) BC Adhesion to Other Aerosol Surfaces[33], (d) Eccentric BC Core-Shell Structure[33]Fig. 3 (a) redistribution structure[67], (b) BC adhesion to other aerosol surfaces[86], (c) BC adhesion to other aerosol surfaces[33], (d) eccentric core-shell structure of BC[33] |
| [1] |
|
| [2] |
|
| [3] |
IPCC. Climate Change 2022: Mitigation of Climate Change., 2022.
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
(冯雪. 南京信息工程大学硕士论文, 2021.).
|
| [111] |
|
| [112] |
(陈超. 南京信息工程大学博士论文, 2018.).
|
| [113] |
|
| [114] |
|
| [115] |
|
/
| 〈 |
|
〉 |