

Critical Issues and Interfacial Design on the Anode Side for Anode-Free Sodium Batteries
Received date: 2024-05-22
Revised date: 2024-09-09
Online published: 2024-09-25
Supported by
National Natural Science Foundation of China(22279164)
Hunan Provincial Science and Technology Plan Projects of China(2022RC3050)
Hunan Provincial Science and Technology Plan Projects of China(2017TP1001)
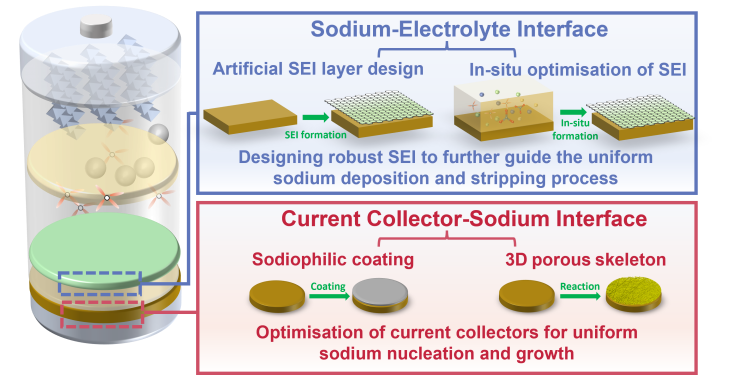
Compared to lithium-ion batteries,sodium-ion batteries have greater advantages in terms of resources,cost,safety,power performance,low-temperature performance,and so on. However,the energy density of sodium-ion batteries is relatively low. To explore broader application prospects,the development of high-specific energy sodium batteries has become a research hotspot in both academia and industry. The anode is considered the key bottleneck constraining the development of the sodium battery industry due to limitations such as the inability of graphite to serve as sodium anodes and the high cost,low Coulombic efficiency,and poor kinetics of mainstream hard carbon materials. In recent years,anode-free sodium batteries (AFSBs) have garnered widespread attention due to their advantages in energy density,process safety,and overall battery cost. However,AFSBs generally show rapid capacity loss due to the rupture of the solid-electrolyte interphase (SEI) layer,increased chemical side reactions,serious dendrite growth and the formation of dead sodium. As the AFSBs operate,active sodium is continuously consumed without additional metallic sodium to replenish it,leading to poor cycling performance and failure of AFSBs. These issues can be attributed to the following characteristics: the high reactivity of sodium,non-uniform nucleation and huge volume expansion. To elucidate the strategies for promoting dendrite-free growth on the anode side of AFSBs,this review focuses on the current collector-sodium interface and sodium-electrolyte interface,including the design of sodiophilic coatings,porous skeleton structure to regulate the sodium nucleation process,and the construction of robust SEI interface,which further guides the homogeneous sodium deposition and stripping process. This systematic review is expected to draw more attention to anode-free configurations and bring new inspiration to the design of high-specific energy batteries.
Contents
1 Introduction
2 Factors affecting sodium deposition on the anode side
2.1 High reactivity of sodium
2.2 Inhomogeneous sodium deposition
2.3 Volumetric deformations
3 Critical differences between sodium and lithium
4 Interface design principles and strategies
4.1 Design principles
4.2 Homogeneous nucleation regulation at the current collector-sodium interface
4.3 Formation of robust SEI at the sodium-electrolyte interface
5 Conclusions and prospects
Jiawen Dai , Chunlin Xie , Rui Zhang , Huanhuan Li , Haiyan Wang . Critical Issues and Interfacial Design on the Anode Side for Anode-Free Sodium Batteries[J]. Progress in Chemistry, 2025 , 37(4) : 551 -563 . DOI: 10.7536/PC240519
表1 Na与Li物理和化学性质比较Table 1 Comparison of physical and chemical properties of Na and Li |
| Parameters | Na | Li |
|---|---|---|
| Atomic radius (Å) | 1.86 | 1.52 |
| Atomic mass (g·mol-1) | 23 | 6.9 |
| Ionic radius (Å) | 1.02 | 0.76 |
| Molar volume (cm3·mol-1) | 23.75 | 12.97 |
| Crustal abundance (%) | 2.3 | 0.0017 |
| Melting point (K) | 371 | 454 |
| Density (g·cm-3) | 0.534 | 0.968 |
| Electron Configurations | [2,8,1] | [2,1] |
| The first ionization energy (KJ·mol-1) | 498.8 | 520.2 |
| Oxidation-reduction potential (V vs SHE) | -2.71 | -3.04 |
| Bulk modulus (GPa) | 6.3 | 11 |
图2 合金化亲钠涂层策略: (a,b) 1 mA·cm-2-1 mAh·cm-2条件下Cu@Bi||Na与Cu||Na沉积剥离库仑效率与Cu@Bi电压曲线;(c) Cu@Bi集流体制备示意图[61];(d) 原位光学显微镜观察Cu及Cu2Sb@Cu在1 mA·cm-2条件下的钠沉积;(e) Cu与Cu2Sn@Cu在1 mA·cm-2-0.5 mAh·cm-2 条件下钠沉积/剥离过程的原位XRD图像;(f) Cu2Sn@Cu钠沉积SEM图像;(g,h) 不同AFSMBs在300 mA·g-1条件下循环稳定性和容量-电压曲线[64];(i) Na2(Sb2/6Te3/6Vac1/6)制备工艺示意图;(j) NST||NVP与Cu||NVP循环性能对比图[66]Fig.2 Strategies for alloying sodiophilic coatin. (a,b) CE of Na plating/stripping for Cu@Bi||Na and Cu||Na at 1 mA·cm-2 and 1 mAh·cm-2 and the voltage profiles of the Cu@Bi. (c) Schematic diagram for the preparation of the Cu@Bi process[61]. Copyright©2023 MDPI. (d) In situ optical microscope surficial observations of sodium deposition on Cu foil and Cu2Sb@Cu at 1 mA·cm-2. (e) In situ XRD patterns of sodium plating/stripping process on copper foil and Cu2Sb@Cu at 1 mA·cm-2 for 0.5 mAh·cm-2. (f) SEM images of sodium deposition on Cu2Sb@Cu. (g,h) Cyclic stability and capacity-voltage curves of different AFSBs at 300 mA·g-1 [64]. Copyright©2024 Wiley‐VCH GmbH. (i) Schematic illustration for preparing Na2(Sb2/6Te3/6Vac1/6). (j) Comparison of cyclic performance of NST|| NVP and Cu|| NVP [66]. Copyright©2021 Wiley‐VCH GmbH |
图3 复合碳化物亲钠层策略: (a) Sn4P3@rGO钠沉积剥离示意图;(b) 0.5 C条件下Na/Sn4P3@rGO||NaVPO4F在各种弯曲状态下循环性能;(c) Na/Sn4P3@rGO||NaVPO4F倍率性能[42] (d) Cu-Cu@C与铜箔钠沉积剥离示意图;(e) Al-Cu@C||NVP/C AFSBs在1 C条件下循环性能图[65]Fig.3 Strategy of Composite Carbide sodiophilic coating. (a) Schematic diagram of sodium deposition and stripping on Sn4P3@rGO. (b) Cycling performance of Na/Sn4P3@rGO||NaVPO4F full cells at 0.5 C under the various bending states. (c) The rate capability of the Na/Sn4P3@rGO|| NaVPO4F full cell at various cycling rates[42]. Copyright©2021 Elsevier. (d) Schematics of Na deposition and stripping on bare Cu and Cu-Cu@C. (e) Cycling performance at 1 C of Al-Cu@C||NVP/C AFSBs[65]. Copyright©2022 Wiley‐VCH GmbH |
图4 其他金属基集流体策略: (a,b) 熔融Na与Zn和Cu的接触角照片;(c) Zn||NVP、Cu||NVP和Al|NVP AFSBs的循环性能图;(d) Zn|| NVP、Cu||NVP和Al||NVP AFSBs在100th循环中的充/放电压曲线[27];(e) 氟化退火Al (F-A-Al)的制备工艺示意图;(f) 钠原子在不同基底上的结合能;(g) 使用不同电解质的P-Al和F-A-Al基底的循环性能[63]Fig.4 Strategies for other metal-based current collectors. (a,b) Photographs showing the contact angles of molten Na on Zn and Cu. (c) Cycling performance of Zn||NVP,Cu||NVP and Al||NVP AFSBs. (d) Charge/discharge voltage profiles of Zn||NVP,Cu||NVP and Al||NVP AFSB for 100th cycle[27]. Copyright©2023 The Royal Society of Chemistry. (e) Schematic diagram of the fabrication process of fluorinated annealed Al (F-A-Al). (f) Binding energies of Na atom on different substrates. (g) Cycling performance with P-Al and F-A-Al substrates using different electrolytes[63]. Copyright © 2023 Wiley‐VCH GmbH |
图5 三维结构铜箔改性策略: (a,b) 二维平面铜箔与三维多孔铜箔钠沉积示意图;(c,d) Na-Cu半电池电化学性能[44];(e) Cu3P@Cu集流体的制备以及钠沉积剥离示意图;(f) Cu3P@Cu||NVP AFSBs循环性能;(g) 充放电曲线;(h) Cu3P@Cu||NVP无负极软包电池在0.5 C下的循环性能[67]Fig.5 Strategies for 3D Copper Foil Structures. Schematic illustration of Na-plating models on different current collectors. (a) 2D planar Cu. (b) 3D porous Cu. (c,d) Electrochemical performance of Na-Cu half cells[44]. Copyright ©2021 Elsevier. (e) The preparation of Cu3P@Cu and Na plating/stripping diagrams. (f) Cycling performance of Cu3P@Cu|| NVP AFSBs. (g) Charge-discharge curves. (h) Cycling performance of Cu3P@Cu||NVP anode-free pouch cells at 0.5 C[67]. Copyright ©2024 Wiley‐VCH GmbH |
图6 碳化物多孔结构策略: (a) 制备的FCTF与钠沉积剥离示意图;(b) FCTF/Na||NVP软包电池循环性能图;(c) FCTF/Na||NVP软包电池倍率性能[69];(d) 真菌处理椴木炭合成过程示意图[68]Fig.6 Strategies for porous carbide structures. (a) The preparation of FCTF and Na plating diagrams. (b) Cycling performance of the FCTF/Na||NVP pouch cell. (c) Rate performance of the FCTF/Na||NVP pouch cell[69]. Copyright©2024 AAAS. (d) Schematic diagram for the synthesis process of fungus-treated basswood carbon[68]. Copyright©2021 American Chemical Society |
图7 人工SEI层策略: (a) 在PB@Cu集流体上构建人工层以及钠沉积剥离的示意图;(b) 1 mA·cm-2-1 mAh·cm-2条件下电压曲线;(c) NVP|| Cu和NVP||PB@Cu全电池的循环稳定性[39];(d) 具有HCOONa修饰铜箔钠沉积示意图;(e) Cu||Na与SF-Cu||Na电池以1 mA·cm-2和1 mAh·cm-2剥离后光学图像;(f) 0.5 C条件下Cu||NVP与SF-Cu||NVP AFSBs循环性能图[70]Fig.7 Strategies for artificial SEI Layer. (a) Schematic illustration of the construction of artificial layer and sodium plating/stripping on PB@Cu current collector. (b) Voltage profiles at 1 mA·cm-2 with a fixed cycling capacity of 1 mAh·cm-2. (c) Cycling stability of NVP||Cu and NVP||PB@Cu AFSBs[39]. Copyright©2021 Chinese Academy of Sciences. (d) Na plating diagrams of AFSBs with a HCOONa-modified Cu foil. (e) Optical image of Cu||NVP and SF-Cu||NVP cells stripped at 1 mA·cm-2 and 1 mAh·cm-2. (f) Cycling properties of the Cu||NVP and SF-Cu||NVP cells at 0.5 C[70]. Copyright©2023 Wiley-VCH GmbH |
图8 原位SEI层优化策略: (a) 常规碳酸盐电解质中循环过程中形成典型的镶嵌SEI;(b) 原位形成Na-Sn合金层与富含NaCl的SEI;(c) Na/Na对称电池在不同浓度SnCl2下的循环性能[41];(d) CO2转化成碳酸盐的可能途径的示意图;(e) Al-C|| NG2410||P2-NCO电池的循环性能;(f) AFSBs循环性能;(g) GCD 40 mA h Al-C||NG2410||P2-NCO软包电池充放电曲线[41];(h) 在电流密度为0.5 A、不同截止电压下,圆柱形AFSB的第三次恒电流放电-充电曲线;(i) 圆柱AFSBs在电流密度为0.5 A时的循环稳定性[73]Fig.8 Strategies for in situ optimisation of SEI layers. (a) Formation of typical mosaic SEI cycled in regular carbonate electrolyte. (b) In situ formed Na-Sn alloy layer plus a NaCl-rich SEI. (c) Cycling performance of Na/Na symmetric cells with various concentrations of SnCl2[41]. Copyright©2019,American Chemical Society. (d) Schematic illustration showing possible routes for generating carbonates from the dissolved CO2. (e) Cycling performance and GCD voltage profiles (inset) of Al-C||NG2410||P2-NCO cell. (f) Cycling performance of AFSBs (Al-C||NG2410||P2-NCO). (g) Charge/discharge curves of pouch cell of AFSBs[71]. Copyright©2023,American Chemical Society. (h) The 3rd galvanostatic discharge-charge plots of the cylindrical AFSBs at current density of 0.5 A with diverse cut-off voltages. i) Cycling stability of the cylindrical AFSBs at a current density of 0.5 A[73]. Copyright©2022 The Author(s),under exclusive licence to Springer Nature Limited |
| [1] |
|
| [2] |
|
| [3] |
(陈锦攀, 陈春晓, 胡志刚. 电池, 2019, 49(1): 79.).
|
| [4] |
|
| [5] |
(闫金定. 航空学报, 2014, 35(10): 2767.).
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
(何菡娜, 王海燕, 唐有根, 刘又年. 化学进展, 2014, 26(4): 572.).
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
(张思伟, 张俊, 吴思达, 吕伟, 康飞宇, 杨全红. 化学学报, 2017, 75(2): 163.).
|
| [23] |
(岳昕阳, 马萃, 包戬, 杨思宇, 陈东, 吴晓京, 周永宁. 物理化学学报, 2021, 37(12): 2.).
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
/
| 〈 |
|
〉 |