

Research Progress of Antiviral Coatings
Received date: 2022-09-19
Revised date: 2022-11-24
Online published: 2023-02-16
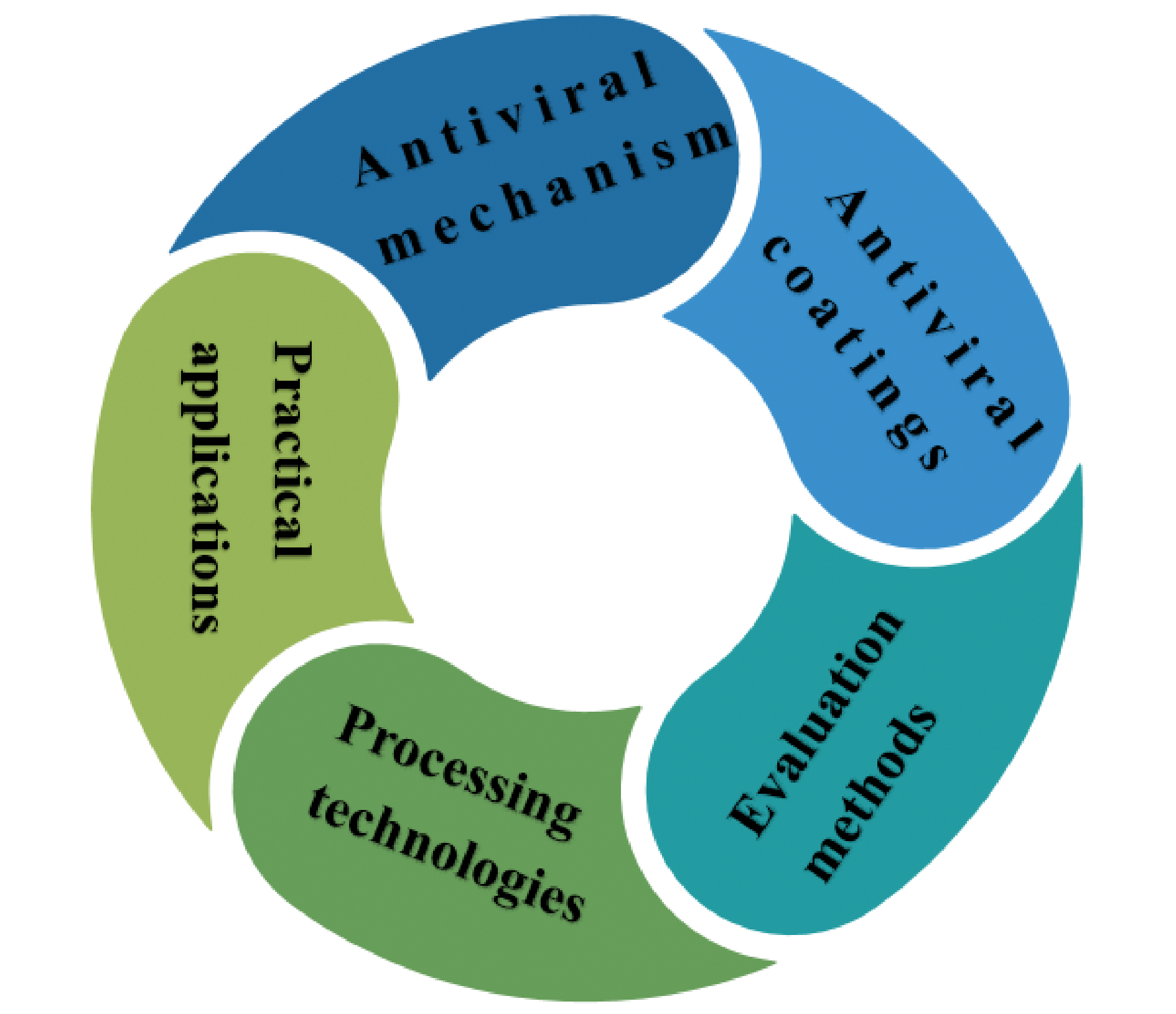
With the large-scale spread of COVID-19 around the world, it has caused serious damage to the health of people around the world. In addition to being transmitted by various droplets, viruses can also be transmitted by human touch of contaminated surfaces. However, as a commonly used surface antiviral method, disinfectants have the disadvantage of discontinuously inactivating viruses, which is bad for inhibiting the spread of various infectious viruses. Therefore, it is urgent to protect the surface of daily objects from virus pollution to eliminate the spread of various respiratory viruses (such as Corona Virus Disease 2019, SARS-CoV-2). From this point of view, it is very important to design and develop effective antiviral coatings. This paper discusses the working mechanisms, performance evaluation methods, processing technologies, practical applications and research progress of nanoparticle antiviral coatings and polymer antiviral coatings for SARS-CoV-2, and also proposes some strategies to design more effective antiviral coatings from the perspective of different types of antiviral coatings. Although some of these antiviral coatings are still in the experimental stage, they still show great potential in the antiviral field.
Liu Jun , Ye Daiyong . Research Progress of Antiviral Coatings[J]. Progress in Chemistry, 2023 , 35(3) : 496 -508 . DOI: 10.7536/PC220917
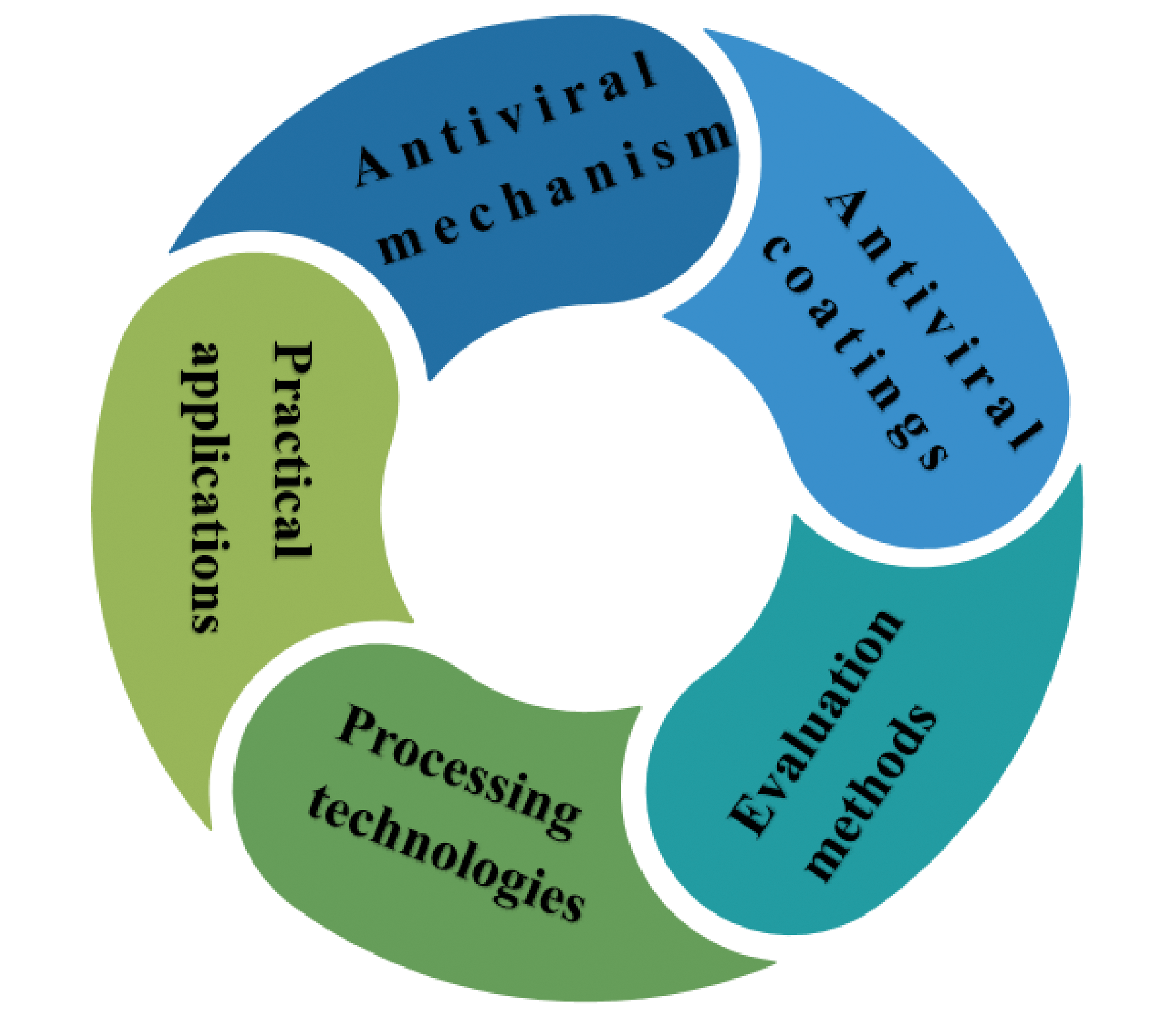
表1 碳基抗病毒纳米材料研究进展Table 1 Progress of carbon-based antiviral nanomaterials |
| Material | Virus | Antiviral effect | ref |
|---|---|---|---|
| Graphene oxide/ Polydimethylsiloxane | HAdV5, HSV-1, CoV | The coating reduces titers of HAdV5 by 1.8 log, HSV-1 by 2.2 log, and CoV by 2.4 log | 32 |
| Polyethylenimine-carbon dots | VSV | Activated by visible light to effectively and efficiently inactivate VSVs | 33 |
| Fullerene derivatives | HIV, Influenza viruses | Promising antiviral activity against HIV and influenza viruses. | 34 |
| Carbon nanotube | SARS-CoV-2 | Exhibit excellent barrier and antiviral effects against SARS-CoV-2 | 35 |
表3 聚合物抗病毒涂层Table 3 Polymer antiviral coatings |
| Material | Virus | Antiviral effect | ref |
|---|---|---|---|
| N,N- dodecyl, methyl PEI | Poliovirus Rotavirus | Approximately 100% virucidal activity | 47 |
| Silane-functionalized polyionenes | SARS-CoV-2 | Exhibiting potent bactericidal (>99.999%) and virucidal (7-log PFU reduction) activities | 48 |
| Surface-Grafted quaternary ammonium polymer | MHV-A59, SuHV-1 | A 4.3-log reduction in infectious MHV-A59 virus and a 3.3-log reduction in infectious SuHV-1 virus | 49 |
| SurfaceWise2 | HCoV-229E, SARS-CoV-2 | The inhibition rate of the two viruses is above 99.9% | 50 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
/
| 〈 |
|
〉 |