

Fabric Coatings Based on Silicon Oxide Structure with both Flame-Retardant and Hydrophobic Properties
Received date: 2023-05-04
Revised date: 2023-08-03
Online published: 2023-09-10
Supported by
Natural Science Foundation of Sichuan Province for Outstanding Youth(2023NSFSC1955)
Science and Technology Program of Yibin(2022GY001)
West Light Talent Program of the Chinese Academy of Sciences(2022~2024)
Tianfu Qingcheng Plan of Sichuan Province(2019~2023)
Open and Innovative Fund of Hubei Three Gorges Laboratory(SK213005)
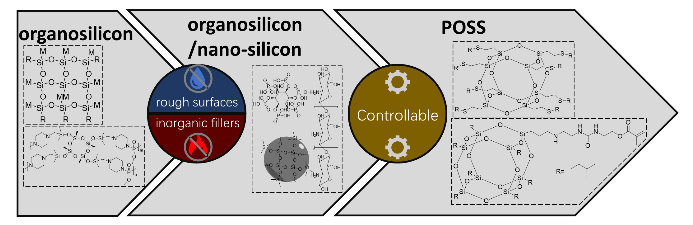
The flame retardancy of coatings on fabrics would always be destroyed and drastically reduced by daily use or routine maintenance because of their hydrophility. So functional coatings with both flame retardancy and hydrophobicity have become a research focus in fabric field. Coatings of silicon oxide compound with high heat resistance and low surface energy have presented good flame retardancy and hydrophobicity. In this paper, the latest research progress about fabric coatings with both excellent flame retardant and hydrophobic properties is described progressively by organosilicon, organosilicon/nano-silicon and polyhedral oligomeric silsesquioxane (POSS) system on the aspects of char formation, low surface energy, micro-nano structure and controllable multi-functionalization. The relationship between the structure of silicon oxide compound and the properties of flame-retardancy and hydrophobicity is deeply investigated. Finally, synergetic mechanism of flame-retardancy and hydrophobicity, enhancement of functional efficiency and service stability of coatings in complex environment are put forward as the future development of fabric coatings with both flame-retardancy and hydrophobicity. According to the requirements of some application scenarios of functional fabric materials, the hot spots are analyzed and prospected.
1 Introduction
2 Research progress
2.1 Organosilicon compounds
2.2 Organosilicon/nano-silicon
2.3 Polyhedral oligomeric silsesquioxane(POSS)
3 Conclusion and outlook
Xinchao Li , Changlin Xia , Mingjun Chen , Ting Wang , Zhicheng Fu , Jinni Deng . Fabric Coatings Based on Silicon Oxide Structure with both Flame-Retardant and Hydrophobic Properties[J]. Progress in Chemistry, 2023 , 35(12) : 1783 -1792 . DOI: 10.7536/PC230501
表1 疏水阻燃涂层的服役稳定性研究Table 1 Durability of hydrophobic flame retardant coating |
| Coating composition | Flame retardance | Hydrophobicity(WCA) | Durability | ref |
|---|---|---|---|---|
| organosilicon compound +nano-SiO2+ DOPO | Burn slowly | Above 150° | 500 cycles adhesion test | 41 |
| phosphate polyurethane + nano-SiO2 + polydimethylsiloxane | LOI is 28.1%, self-extinguishing | 160° | undergoing 1000 cycles of abrasion, 60 min of ultrasonic washing and 50 standard machine washing cycles | 42 |
| flame retardancy-dopamine + silver-polydimethylsiloxane | LOI is 29.8%, and self-extinguishing | Above 150° | 50 standard washing cycles | 65 |
| fluoroalkyl silanes + Sb2O5 + Al(OH)3 | LOI is 45.1%, and self-extinguishing | 152° | 1000 washing cycles | 66 |
| N,N-dimethyloctadecyl phosphate acrylamide | LOI is 21%, and self-extinguishing | 157° | 30 laundering cycles | 67 |
| diethylenetriamine penta(methylene- phosphonic acid) + Fe3+ | self-extinguishing | 155.6° | 12 laundering cycles | 68 |
| Nano-SiO2 + APP + fluorinated alkyl silane | self-extinguishing | 156° | 20 cyclic cross hatch tape peel tests, 12 cycles cyclic abrasion, 30 cycles tape-peeling and 30 washing cycles | 69 |
| [1] |
|
| [2] |
(欧育湘. 实用阻燃技术. 北京: 化学工业出版社, 2002.).
|
| [3] |
(卢林刚, 陈淮思, 曹飞, 董希琳, 唐凯. 化学学报, 2010, 68(15): 1532.).
|
| [4] |
(王晓伟, 刘景富, 阴永光. 化学进展, 2010, 22(10): 1983.).
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
/
| 〈 |
|
〉 |