

Molecular Electronic Devices Based on Carbon Electrodes
Received date: 2023-06-21
Revised date: 2023-08-11
Online published: 2023-09-10
Supported by
National Natural Science Foundation of China(21773169)
National Natural Science Foundation of China(21973069)
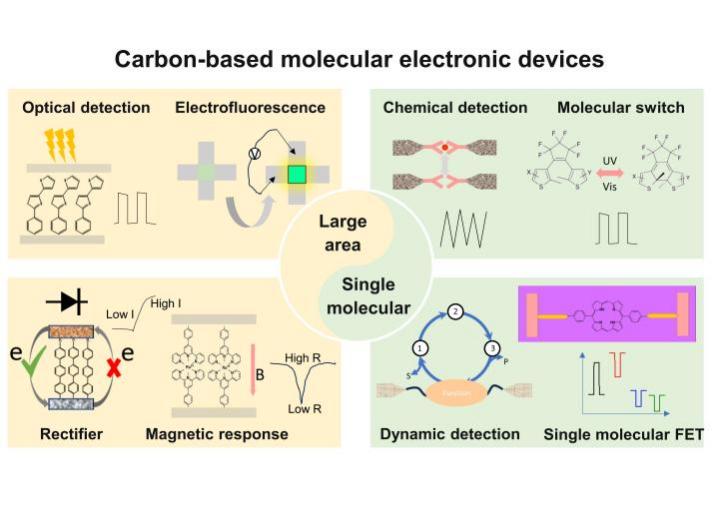
Molecule-based electronic devices, using the intrinsic electronic structure of molecules as device units and constructing electronic devices at the molecular scale, serve as an ideal experimental platform for studying molecular charge transfer mechanisms. They also provide a novel strategy for achieving new functional electronic devices at the micro-nano scale. The realization of a micro-nano electrode gap and a reliable electrode-molecule connection are key factors in developing highly reproducible molecular devices. Carbon materials have been widely applied in the construction of molecular devices due to their remarkable chemical stability and abundant surface chemistry. This review summarizes the research status of using carbon as electrodes in molecular device construction, showcasing the prominent advantages of carbon materials, such as high stability, low cost, and scalability, as well as their applications and research progress in large-area molecular devices and single-molecule devices. The review presents a wealth of achievements in the construction of functional molecular devices, such as molecular switches and rectifiers, using carbon electrodes, as well as the study of the structure-performance relationship in molecular-electron transport. Lastly, this work analyzes the challenges currently faced in carbon-based molecular device research and provides prospects for the chemical connection of carbon electrode-molecular interface and functionalization of carbon-based molecular devices, as well as the integration of future molecular devices.
1 Introduction
2 Electrode materials in molecular junctions
2.1 Metal electrodes
2.2 Semiconductor electrodes
2.3 Carbon electrodes
3 Carbon electrodes in large-area molecular devices
4 Carbon electrodes in single molecular junctions
4.1 Preparation technology of carbon-based single molecular junctions
4.2 Function and regulation of carbon-based single molecular junctions
4.3 Analysis and detection of carbon-based single molecule devices
5 Conclusion and outlook
Junhong Xue , Xuan Ji , Cong Chen , Xiaohai Ding , Xi Yu , Wenping Hu . Molecular Electronic Devices Based on Carbon Electrodes[J]. Progress in Chemistry, 2024 , 36(1) : 1 -17 . DOI: 10.7536/PC230618
图2 (a) STM-BJ装置原理图;(b) STM-BJ测量过程示意图及相应的电导变化曲线[40];(c) 左:AgTS/SAM/Ga2O3/EGaIn分子结的光学显微镜图像;右:相应器件垂直结构示意图[41]Fig. 2 (a) Schematic of STM-BJ set-up; (b) Schematic diagram of the STM-BJ measurement process and corresponding conductance change curves[40]. Copyright 2003, AAAS; (c) Left: optical microscope image of AgTS/SAM/Ga2O3/EGaIn molecular junctions (MJs); Right: vertical structure diagram of corresponding device[41]. Copyright 2013, Springer Nature |
图3 (a) n-Si(111)/molecular/Hg分子整流器的示意图;(b) n-Si(111)/molecular/Hg分子结的J-V曲线(绿色:对溴代苯乙烯,蓝色:苯乙烯,黑色:对甲基苯乙烯)[44]Fig. 3 (a) Schematic diagram of n-Si(111)/molecular/Hg molecular rectifier; (b) J-V curves for n-Si(111)/molecular/Hg molecular junctions (green: Br-styrene, blue: H-styrene, black: CH3-styrene) [44]. Copyright 2013, Wiley |
表1 不同碳材料的相关物性比较Table 1 Comparison of correlation properties of different carbon materials |
| Type of material | Material density (g·cm-3) | d002 (Å) | ρ (Ω·cm) | ref |
|---|---|---|---|---|
| HOPG, a-axis | 2.26 | 3.354 | 4×10-5 | 51 |
| HOPG, c-axis | 2.26 | 3.354 | 0.17 | 52 |
| Disordered graphite | 1.80 | 3.350 | 1×10-3 | 53 |
| Tokai GC-2000℃ | 1.50 | 3.480 | 4.2×10-3 | 54 |
| Carbon fiber | 1.80 | 3.400 | (5~20)×10-4 | 55 |
| Evaporation of amorphous carbon | 2.00 | >3.4000 | ~103 | 56 |
| Hydrogenated amorphous carbon | 1.4~1.8 | - | 107~1016 | 57 |
| PPF | - | - | 0.006 | 58 |
| B-doped diamond | - | - | 0.05~0.5 | 59 |
图4 (a) 通过共价酰胺键连接的单分子结示意图;(b) 通过π-π相互作用结合的单分子结示意图;(c) 通过碳碳共价键连接的大面积分子结示意图;(d) 通过π-π相互作用结合的大面积分子结示意图Fig. 4 (a) Schematic diagram of a single molecular junction connected by a covalent amide bond; (b) Schematic diagram of a single molecular junction bound by π-π interaction; (c) Schematic diagram of large molecular junctions connected by carbon-carbon covalent bonds; (d) Schematic diagram of large molecular junctions bound by π-π interaction |
图5 (a) 上:芳香胺原位重氮化在碳电极表面接枝过程示意图[27],下:部分用于电接枝修饰碳表面的化学结构[65]; (b) 单晶石墨材料的态密度分布(下图为费米能级处态密度的放大图)[51]Fig. 5 (a) Upper: Schematic diagram of the grafting process of the aromatic amine in situ diazotization on the surface of the carbon electrode[27]. Copyright 2020, MDPI, Basel, Switzerland; Lower: part representative chemical structure used for electrical grafting to modify carbon electrodes[65]. Copyright 2020, The Royal Society of Chemistry; (b) State density distribution of a single crystal graphite material (the figure on the right is an enlarged view of state density at the Fermi level) [51]. Copyright 2008, American Chemical Society |
图7 (a) 在直径100 mm晶圆上基于eC和电接枝制备的碳基分子电子芯片光学图像以及其局部放大分子结的示意图;(b) 柔性衬底上碳基器件的弯曲光学图像及单个分子结弯曲状态下的结构示意图[59]Fig.7 (a) Optical image of a carbon-based molecular electronic chip prepared by eC and electrical grafting on a 100 mm diameter wafer and schematic diagram of its locally amplified molecular junction; (b) Bending optical image of a carbon-based device on a flexible substrate and schematic diagram of a single molecular junction in bending state[59]. Copyright 2016, American Chemical Society |
图8 (a) 开路电势(OCP)测量装置示意图;(b) BTB分子结示意图(底部:石英,顶部:碳);(c) 观察407 nm二极管激光上下照射BTB分子结三个开/关光周期的OCP[80];(d) Au/eC/Ru(bpy)3/eC/Au分子器件示意图及其施加电压前后分子结的光学图像;(e) Ru(bpy)3(12.8 nm)分子结的紫外吸收、光电流和发光光谱叠加图;(f) Ru(bpy)3(12.8 nm)分子结在乙腈蒸气下,3.2 V的偏置脉冲和静置几分钟的MJ重复脉冲的总光发射与时间的关系[9]Fig. 8 (a) Schematic diagram of OCP measuring apparatus; (b) Schematic diagram of BTB MJ (Bottom: quartz, top: carbon); (c) Observed OCP for three on/off light cycles for BTB MJ with top and bottom illumination by a 407 nm diode laser[80]. Copyright 2018, American Chemical Society; (d) Schematic diagram of Au/eC/Ru(bpy)3/eC/Au molecular junction and optical image of molecular junction before and after voltage application; (e) Overlay of UV absorption, photocurrent and luminescence spectra of Ru(bpy)3(12.8 nm) MJs; (f) Total light emission versus time under acetonitrile vapor for bias pulses of 3.2 V and repeated pulses for Ru(bpy)3(12.8 nm) MJ at rest for several minutes after a bias pulse[9]. Copyright 2019, American Chemical Society |
图9 (a)左:Au/a-C/Ru(tpy)2/a-C/Au分子结的示意图,右:分子结内双极化子电荷输运机理示意图(a-C:无定形碳膜);(b) Au/a-C/Ru(tpy)2/a-C/Au分子结的磁响应曲线[84]Fig. 9 (a) Left: Schematic diagram of the Au/a-C/Ru(tpy)2/a-C/Au molecular junctions, Right: schematic diagram of charge transport mechanism of bipolaron in molecular junction(a-C: amorphous carbon film); (b) Magnetic response curve of Au/a-C/Ru(tpy)2/a-C/Au molecular junction[84]. Copyright 2022, Wiley |
图10 制备碳纳米管纳米电极示意图[90]:(a) 电烧结法;(b) 氧等离子体氧化切割法;(c) 聚焦电子束切割法;(d) 电烧结法制备石墨烯纳米电极示意图[64];(e) 锯齿状石墨烯点接触阵列的制备,左:氧等离子体通过电子束光刻定义的锯齿状PMMA窗口精确切割石墨烯片的示意图,右:通过氧化切割形成的锯齿状石墨烯触点被羧酸端基功能化并且分离仅有几纳米的示意图[91];(f) 机械控制断结和石墨烯/单富勒烯/石墨烯结示意图以及C60、C70、C76和C90的化学结构[92]Fig. 10 Schematic diagram of preparing carbon nanotube nanoelectrodes[90]. Copyright 2022, IOP Publishing. (a) Electrical breakdown method; (b) Oxygen plasma oxidation cutting method; (c) Focused electron beam cutting; (d) Schematic diagram of preparation of graphene nanoelectrodes by Electrical breakdown [64]. Copyright 2011, American Chemical Society; (e) Fabrication of indented graphene point contact arrays. Left: schematic illustration of an oxygen plasma precisely cutting a graphene sheets through an indented PMMA window defined by electron-beam lithography. Right: schematic illustration of indented graphene point contacts formed by oxidative cutting were functionalized by carboxylic acid end groups and separated by as little as a few nanometres. [91]. Copyright 2012, Wiley; (f) Schematic of mechanically controlled break junction and graphene/single-fullerene/graphene junction and chemical structure of C60, C70, C76, and C90[92]. Copyright 2019, Springer Nature |
图 11 (a) 石墨烯-二芳基乙烯-石墨烯结的示意图;(b) 紫外可见光辐射下,二芳基乙烯分子在打开和关闭形式下可逆切换引起的实时测量电流,VD =100 mV,VG =0 V[89];(c) 左:CNTB-M/CNTT vdWI器件的三维图,右:依据电场开关CNTB-M/CNTT vdWI中基于反式和顺式异构体的分子极化变化的DFT模拟模型;(d) CNTB-M/CNTT vdWI器件的Ids-Vds曲线(分子在顺反状态间切换产生存储窗口)[99]Fig.11 (a) Schematic diagram of a graphene-diarylethene-graphene junction; (b) Real-time measurement of the current through a diarylethene molecule that reversibly switches between the closed and open forms, upon exposure to UV and Vis radiation, respectively. VD =100 mV and VG =0 V[89]. Copyright 2016, AAAS; (c) Left: three-dimensional view of the CNTB-M/CNTT vdWI device, Right: DFT simulation model of the molecule polarization change based on trans and cis isomers in CNTB-M/CNTT vdWI according to electrical switching. (d) Ids-Vds curves of CNTB-M/CNTT vdWI device (the switching of molecules between trans and cis states leads to the generation of a memory window)[99]. Copyright 2022, Springer Nature |
图 12 (a) 石墨烯-卟啉-石墨烯结示意图,突出离子液体门控和氢互变异构;(b) 通过卟啉分子的实时电流测量,在VD=950 mV时,四个态之间的转变;(c) 在VD= -0.02、-0.1、-0.2、-0.3和-0.4 V下器件的转移特性(插图展示了偏压依赖的开关比)[101];(d) 跨平面断裂结装置和外加电场作用下3个M-2D-vdWHs分子结内构象演变示意图;(e) 左:TPA M-2D-vdWHs分别在100、200和300 mV偏压下的1D电导直方图,插图给出了典型的电导-位移轨迹;右:TPA随电场的演变示意图,从具有高电导率的TWP构象(ON状态)到具有低电导率的TC构象(OFF状态);(f) 电场在100和300 mV之间切换时TPA M-2D-vdWHs的可逆开关[97]Fig. 12 (a) Schematic of a graphene-porphyrin-graphene junction that highlights ionic liquid gating and hydrogen tautomerization; (b) Real-time measurement of the current through a porphyrin molecule that highlights the transitions between four states at 950 mV; (c) Transfer characteristics at VD= -0.02, -0.1, -0.2, -0.3, and -0.4 V (Inset shows VD-dependent on/off ratios)[101]. Copyright 2022, AAAS; (d) Schematic diagram of the XP-BJ setup and the conformational evolution of three M-2D-vdWHs under the applied electric field; (e) Left: The 1D conductance histograms for TPA M-2D-vdWHs under the bias of 100, 200, and 300 mV, respectively. The inserts give the typical conductance-displacement traces, Right: Schematic evolution of TPA along with the electric field, from a TWP conformation with a high conductivity (ON state) to a TC conformation with a low conductivity (OFF state); (f) Reversible switching of TPA M-2D-vdWHs as the electric field was switched between 100 and 300 mV[97]. Copyright 2023, AAAS |
图 13 (a) 单分子器件在单分子水平上检测蛋白质的传感机制示意图;(b) 当凝血酶和盐酸胍交替处理时,同一器件的三个代表性开关周期,VSD = -50 mV[104]Fig. 13 (a) Schematic representation of the sensing mechanism showing single-molecule devices detect proteins at the single-molecule level; (b) Three representative switching cycles for the same device when alternately treated with thrombin and guanidine HCl, VSD = -50 mV[104]. Copyright 2011, Wiley |
图14 (a)监测单分子催化循环过程的策略及其器件结构的示意图,在电流中可以检测到催化功能中心的实时变化;(b) 通过催化功能中心的实时变化能够在电流中被检测,突出强调一个催化循环过程中四个导电态的转变[106]Fig.14 (a) Schematic strategy of the monitoring process for a single-molecule catalytic cycle and device structure; (b) Real-time measurement of the current through catalyst functional unit that highlights the transitions between four conductance states of one catalytic cycle[106]. Copyright 2021, Springer Nature |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
/
| 〈 |
|
〉 |