

Saccharide Sensors Based on Phenylboronic Acid Derivatives
Received date: 2023-05-19
Revised date: 2023-08-26
Online published: 2023-12-10
Supported by
National Natural Science Foundation of China(22278064)
National Natural Science Foundation of China(21878040)
National Natural Science Foundation of China(22238002)
Fundamental Research Funds for the Central Universities(DUT22LAB610)
Research and Innovation Team Project of Dalian University of Technology(DUT2022TB10)
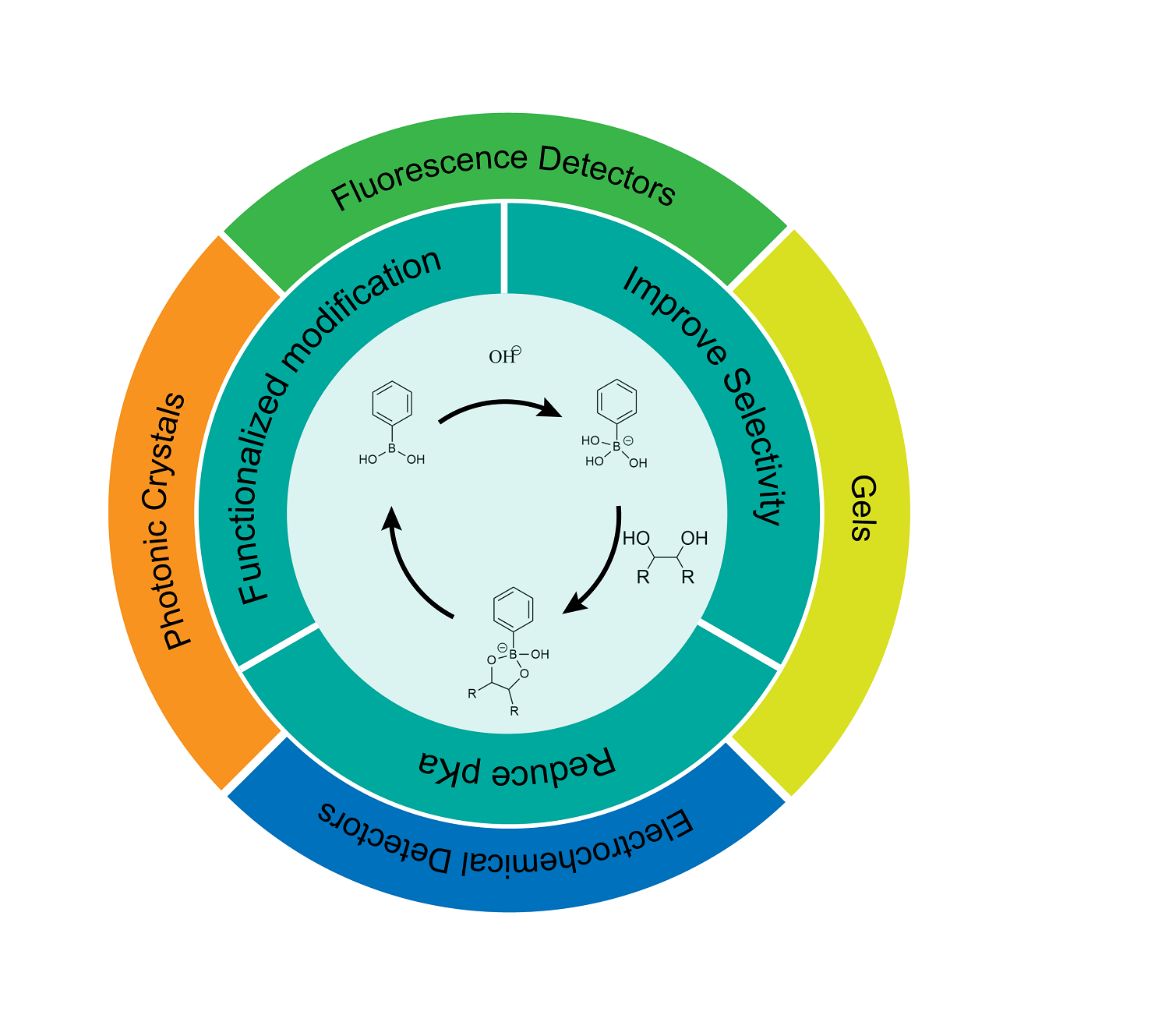
Phenylboronic acid, a kind of synthetic molecule that can covalently bind with saccharide, has attracted wide attention in the field of saccharide detection. It has the characteristics of good stability, strong recognition ability and easy coupling with various detection systems. In this paper, the mechanism of phenylboronic acid binding to saccharide and its specific applications in detection was first introduced. What’s more, the strategies for structural modification, in the manner of introducing electron-withdrawing group or electron-donating group into ortho, meta and para position of the boric acid group on the benzene ring, were mainly discussed, and the progress made in reducing pKa and improving the selectivity according to these strategies were summarized. At the same time, the saccharide sensors based on these new phenylboronic acid derivatives in recent years were also summarized, including electrochemical sensors, fluorescence sensors, gels/microgels and photonic crystals, and their detection principles were discussed. The main analytes are monosaccharides with similar structures, such as glucose and fructose. Finally, the research of these sensors based on phenylboronic acid derivatives was compared, and their advantages and disadvantages were analyzed. Meanwhile, the applications of saccharide sensors based on phenylboronic acid derivatives in the future are prospected from two aspects including the integration of diagnosis and treatment and the identification of saccharide in complex chemical environment.
1 Introduction
2 Phenylboronic acid and its derivatives
2.1 Reaction principle of phenylboronic acid and saccharides
2.2 Structural modification strategy of phenylboronic acid
2.3 Detection principle of saccharides in phenylboronic acid
3 Saccharide sensors based on phenylboronic acid derivatives
3.1 Electrochemical sensors for saccharide detections
3.2 Fluorescent sensors for saccharide detections
3.3 Photonic crystals for saccharide detections
3.4 Gels for saccharide detections
4 Conclusion and outlook
Key words: phenylboronic acid; saccharide; electrochemistry; fluorescence; gel; photonic crystal
Tan Shi , Donghui Kou , Yanan Xue , Shufen Zhang , Wei Ma . Saccharide Sensors Based on Phenylboronic Acid Derivatives[J]. Progress in Chemistry, 2024 , 36(1) : 106 -119 . DOI: 10.7536/PC230519
图2 (a)常见吸电子基改性苯硼酸衍生物的分子结构;(b)邻位给电子基改性苯硼酸衍生物的分子结构;(c)2-丙烯酰胺基苯硼酸的分子结构;(d)常见二元苯硼酸衍生物的分子结构Fig. 2 (a) Molecular structure of common electron- withdrawing modified phenylboronic acid derivatives;(b) molecular structure of ortho-electron-donating modified boronic acid derivatives; (c) molecular structure of 2-acrylamidophenylboronic acid; (d) molecular structure of common dibasic phenylboronic acid derivatives |
图6 氧化还原改性后的4-二茂铁-苯硼酸(4-Fc-PBA)/天然β-环糊精(β-CDs)复合物(a)检测果糖的机理;(b)基于氧化峰和(c) 基于还原峰的校准线(果糖浓度高达5 mM)[62]Fig. 6 (a) Mechanism of fructose detection by 4-Ferrocene- phenylboronicacid (4-Fc-PBA)/natural β-cyclodextrins (β-CDs) calibration lines based on (b) oxidation peaks and on (c) reduction peaks (fructose concentration up to 5 mM)[62] |
图8 在pH值为8的PBS溶液中,恢复型竞争反应类荧光复合探针(5 mg·mL−1)对不同浓度葡萄糖响应的荧光光谱(插图: 复合探针的(F−F0)/F0对葡萄糖浓度的半对数图)[73]Fig. 8 Fluorescence response of composite probe (5 mg·mL−1) upon addition of various concentrations of glucose in a pH 8 PBS solution. Inset: semilogarithmic plot of (F−F0)/F0 of composite probe vs the concentration of glucose [73] |
图 10 苯硼酸改性复合荧光探针(a) 1F/γ-CyD和(b) 2N/γ-CyD在不同糖浓度(D-葡萄糖、D-果糖、D-半乳糖和L-葡萄糖)的二甲基亚砜(DMSO)/水中时在494 nm处的荧光强度(1F/γ-CyD和2N/γ-CyD的激发波长分别为328和378 nm)[83]Fig. 10 Fluorescence intensities at 494 nm of 1F/γ-CyD (a) and 2N/γ-CyD (b) at various concentrations of saccharides (D-glucose, D-fructose, D-galactose, and L-glucose) in DMSO/water (2/98 in v/v) (The excitation wavelengths were set as 328 nm for 1F/γ-CyD and 374 nm for 2N/γ-CyD)[83] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
(王卓, 李朔. 大学化学, 2020, 35(7): 95.).
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
/
| 〈 |
|
〉 |