

Ball-Milled Click Chemistry: A Solvent-Free Green Chemistry
† These authors contributed equally to this work.
Received date: 2023-07-17
Revised date: 2023-09-15
Online published: 2024-02-26
Supported by
National Natural Science Foundation of China(52103005)
Liaoning Revitalization Talents Program(XLYC2203072)
startup funding of Liaoning Petrochemical University(2021XJJL-003)
Click chemistry won the Noble Prize in 2022 due to easy synthesis, high selectivity, single product, and no toxic side product. Click chemistry was originally designed as green chemistry to work in aqueous solutions or environmentally friendly organic solvents. However, due to the poor solubility of reactants, polar and toxic solvents are usually required to use. The solvent used violates the concept of green chemistry, as well as increases the cost. These issues hinder click chemistry to be a state-of-art green chemistry. One of the solutions to optimize click chemistry is to avoid using any solvent. Herein, ball-milled mechanochemistry does not limit reactants’ solubility and could avoid solvent use. Ball-milled mechanochemistry is a new kind of chemical reaction that is conducted in a ball mill, is induced by mechanical force, and needs no solvent or a minimal amount of solvent. As a new way of organic synthesis, ball-milled mechanochemistry could easily achieve the low-energy carbon-heteroatom bonds, which constitute the linkages in click chemistry. Therefore, it could integrate with click chemistry and achieves ball-milled click chemistry. In comparison to traditional solution click chemistry, ball-milled click chemistry avoids solvent use. Moreover, it is even superior in the ways that the reaction time is shortened, the reaction temperature is lowered, and the catalyst used is simplified. In this review, ball-milled click chemistry examples are reported as much as the authors can find, including CuAAc, Diels-Alder, amine and isothiocyanate reactions, amine thiol reactions, and nitroxide radical coupling reactions. To provide readers with a better ball-milled click chemistry manual, this paper also contains ball mill machine choice guidance, liquid-assisted grinding choice guidance, and factors impacting ball-milled click chemistry conversion, including catalyst choice, additive choice, ball choice, stoichiometry, and milling time.
1 Introduction
1.1 Ball mill machines
1.2 Liquid/solid assisted grinding
2 Ball-milled click chemistry
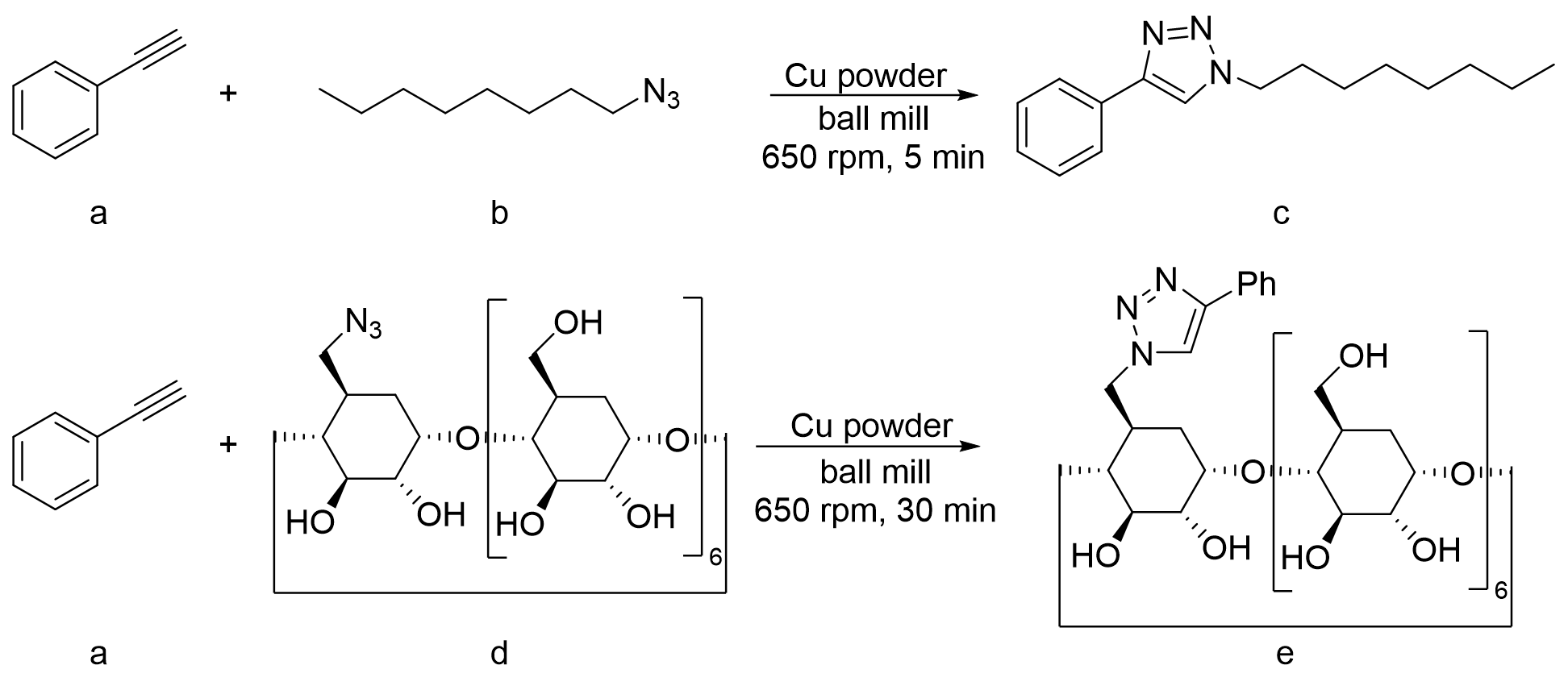
2.1 Ball-milled CuAAc
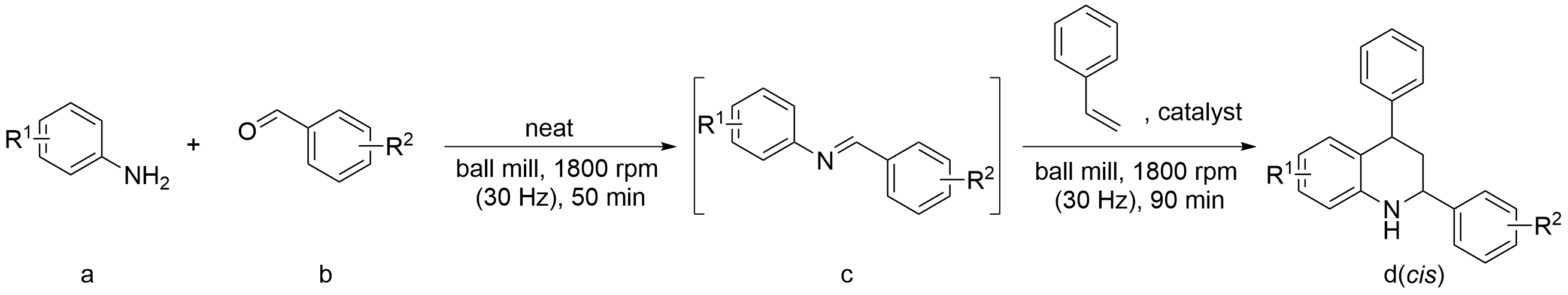
2.2 Ball-milled Diels-Alder
2.3 Ball-milled amine and isothiocyanate reactions
2.4 Ball-milled amine thiol reactions
2.5 Ball-milled nitroxide radical coupling reactions
3 Factors impacting ball-milled click chemistry
3.1 Catalysts
3.2 Milling balls
3.3 Additive
3.4 Stoichiometry
3.5 Reaction time
4 Conclusion and outlook
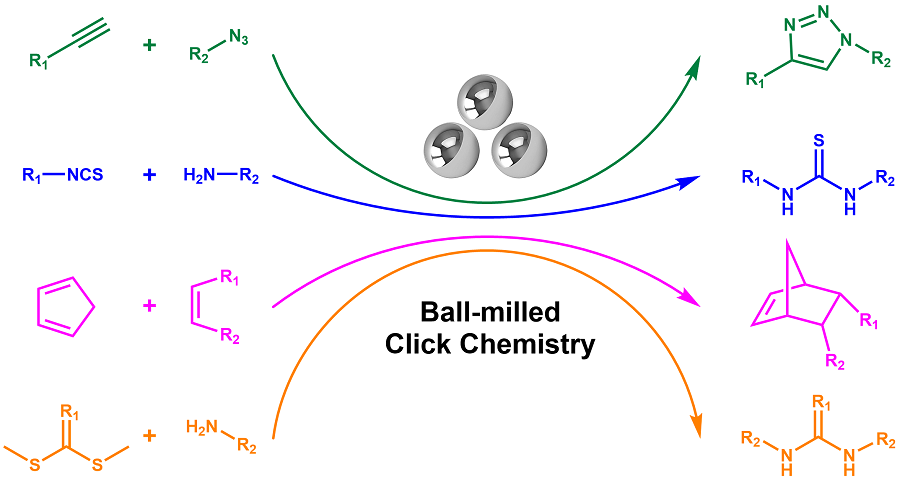
Xinqi Guan , Yuan Sang , Hailing Liu . Ball-Milled Click Chemistry: A Solvent-Free Green Chemistry[J]. Progress in Chemistry, 2024 , 36(3) : 401 -415 . DOI: 10.7536/PC230711
表1 震动球磨机、行星球磨机和搅拌球磨机适用情况Table 1 Introduction of mixer ball mill, planetary ball mill, and stir ball mill |
| Mixer ball mill | Planetary ball mill | Stir Ball mill | |
|---|---|---|---|
| Mechanical force | Impact force | Shear force | - |
| Common Brand | Retsch | Fritsch | Outotec HIGMill |
| Invented time | 1923 | 1961 | 1922 |
| Scale | grams | 10~100 grams | More than a ton |
| Application | Inorganic chemistry, organic chemistry | Organic Chemistry | Physical grinding |
表2 球磨-CuAAc反应与溶液-CuAAc产率对比Table 2 Yields from Ball-milled CuAAc versus CuAAc in solution reaction |
| Product | Catalyst | Yields from solution reaction[%]a | Yields from ball mill[%]b |
|---|---|---|---|
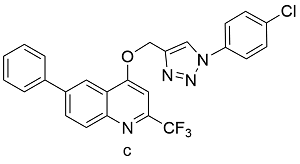 | Cu(OAc)2 | 21 | 57 |
| CuI | 5 | 85 | |
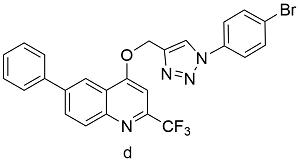 | Cu(OAc)2 | 45 | 60 |
| CuI | 40 | 87 | |
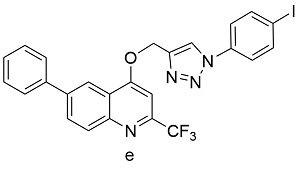 | Cu(OAc)2 | 89 | 77 |
| CuI | 52 | 92 | |
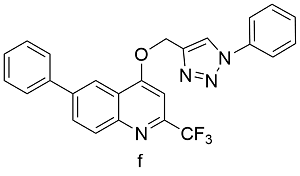 | Cu(OAc)2 | 10 | 72 |
| CuI | 5 | 79 |
aSolution reaction condition:3.5 h,60℃. Methanol solvent was used for Cu(OAc)2;CuI, N,N-Diisopropylethylamine was used as the additive, CH2Cl2 was used as the solvent; bBall mill reaction condition:3.5 h,room temperature,two 7 mm stainless steel balls, PTFE milling jar, 30 Hz. Cu(OAc)2 was used without additives or solvents; CuI was used with N,N-Diisopropylethylamine and acetic acid |
表3 醋酸铜、碘化铜、黄铜球的催化下,球磨CuAAc的反应产率Table 3 Ball-milled CuAAc yields catalyzed by Cu(OAc)2, CuI, or brass ball |
| Product | Catalyst | Yields[%] |
|---|---|---|
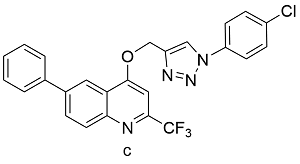 | Cu(OAc)2a | 57 |
| CuIb | 85 | |
| Brass ballc | 77 | |
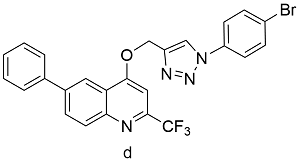 | Cu(OAc)2a | 60 |
| CuIb | 87 | |
| Brass ballc | 80 | |
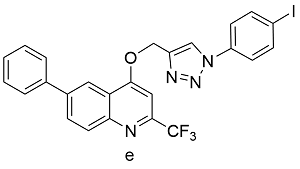 | Cu(OAc)2a | 77 |
| CuIb | 92 | |
| Brass ballc | 87 | |
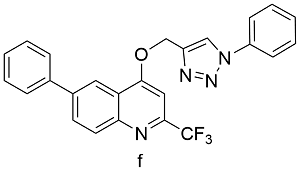 | Cu(OAc)2a | 72 |
| CuIb | 79 | |
| Brass ballc | 76 |
aReaction condition:Cu(OAc)2, two 7 mm stainless steel balls, PTFE milling jar, 30 Hz, 3.5 h, room temperature; breaction condition: CuI, N,N-Diisopropylethylamine, acetic acid, two 7 mm stainless steel balls, PTFE milling jar, 30 Hz, 3.5 h,room temperature; creaction condition: N,N-Diisopropylethylamine, acetic acid, two 7 mm brass balls, PTFE milling jar, 30 Hz, 3.5 h, room temperature |
表4 一锅法球磨CuAAc在不定量的催化剂下的产率Table 4 One pot ball-milled CuAAc yields under varied catalyst dose |
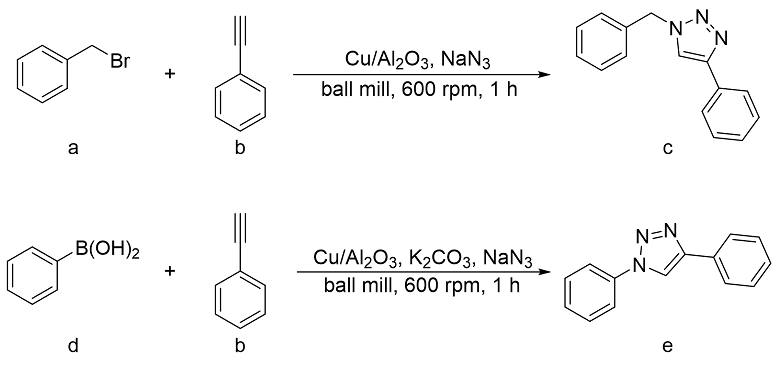 | ||
|---|---|---|
| Number | Catalyst amount (mol%) | Yields(%) |
| 1 | 0 | 0 |
| 2 | 2 | 51 |
| 3 | 5 | 72 |
| 4 | 10 | 96 |
表5 球磨Diels-Alder反应中Lewis酸和Bronsted酸催化剂对产率的影响Table 5 Ball-milled Diels-Alder reaction yields catalyzed by Lewis acids or Bronsted acids |
 | ||
|---|---|---|
| Number | Catalyst | Yields(%) |
| 1 | ZnCl2 | 76 |
| 2 | AlCl3 | 85 |
| 3 | CuCl2 | 15 |
| 4 | FeCl3 | 86 |
| 5 | Cu(OAc)2 | 10 |
| 6 | Co(OAc)2 | Not detected |
| 7 | Mn(OAc)2 | Trace |
| 8 | Pd(OAc)2 | Trace |
| 9 | BF3·OEt2 | 81 |
| 10 | Cu(OTf)2 | 56 |
| 11 | TFA | 48 |
| 12 | p-TsOH | 12 |
| 13 | KHSO4 | Trace |
| 14 | KH2PO4 | Not detected |
表6 相同总质量,不同大小和数量研磨球对反应的影响Table 6 Yields from different mill balls in terms of sizes and numbers |
 | |||||
|---|---|---|---|---|---|
| Number | Ball numbers | Effective surface area a(mm2) | Yields (%) | ||
| 2mm Ø | 5mm Ø | 10mm Ø | |||
| 1 | 0 | 0 | 10 | 10666 | 67 |
| 2 | 625 | 0 | 10 | 18520 | 80 |
| 3 | 1500 | 48 | 0 | 30144 | 99 |
aEffective surface area=surface area of balls+surface area of the jar |
表7 一锅法球磨CuAAC在不定量K2CO3添加剂下的产率Table 7 One pot ball-milled CuAAC yields with varied K2CO3 additives |
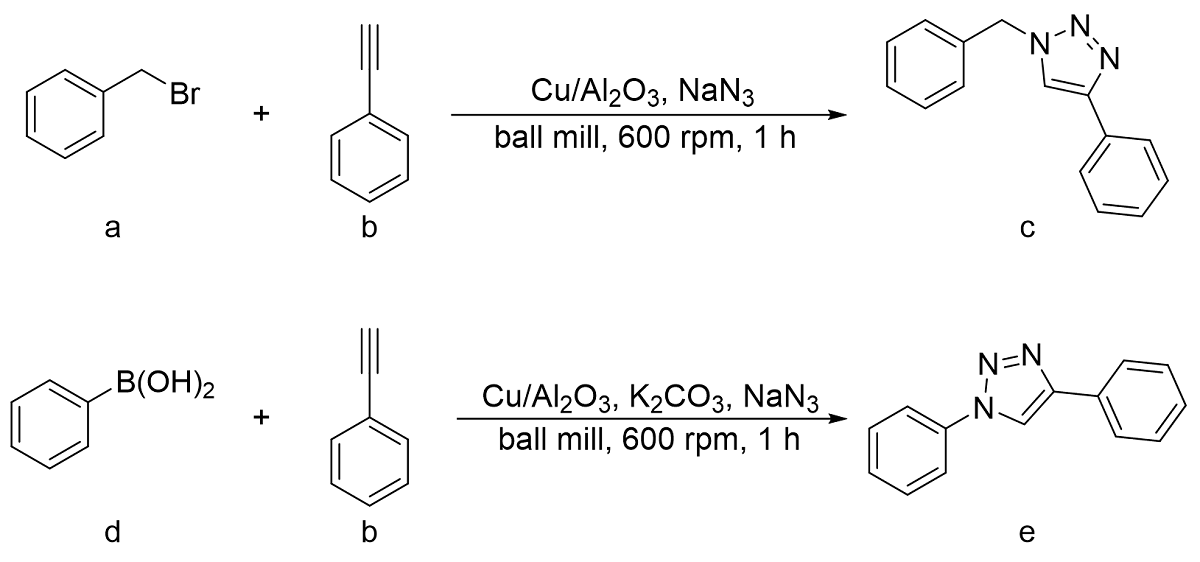 | ||
|---|---|---|
| Number | K2CO3(equivalent) | Yields(%) |
| 1 | 0 | 52 |
| 2 | 1 | 91 |
| 3 | 3 | 90 |
表8 球磨CuAAc中试剂比例对产率的影响Table 8 Ball-milled CuAAc yields impact by stoichiometric ratio |
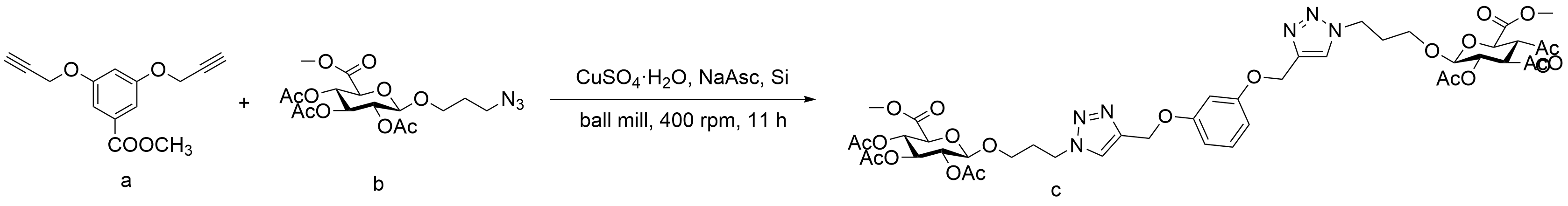 | |||
|---|---|---|---|
| Number | Stoichiometry a:b:CuSO4·H2O:NaAsc | Reaction time | Conversion(%) |
| 1 | 0.5:1.1:0.2:0.35 | 9 | 54 |
| 2 | 0.5:1.65:0.3:0.53 | 9 | 61 |
| 3 | 0.5:2.5:0.3:0.53 | 11 | 65 |
| 4 | 0.5:3.5:0.4:0.7 | 11 | >99 |
表9 一锅法球磨CuAAc在不同反应时间下的产率Table 9 One pot ball-milled CuAAc yields under varied reaction time |
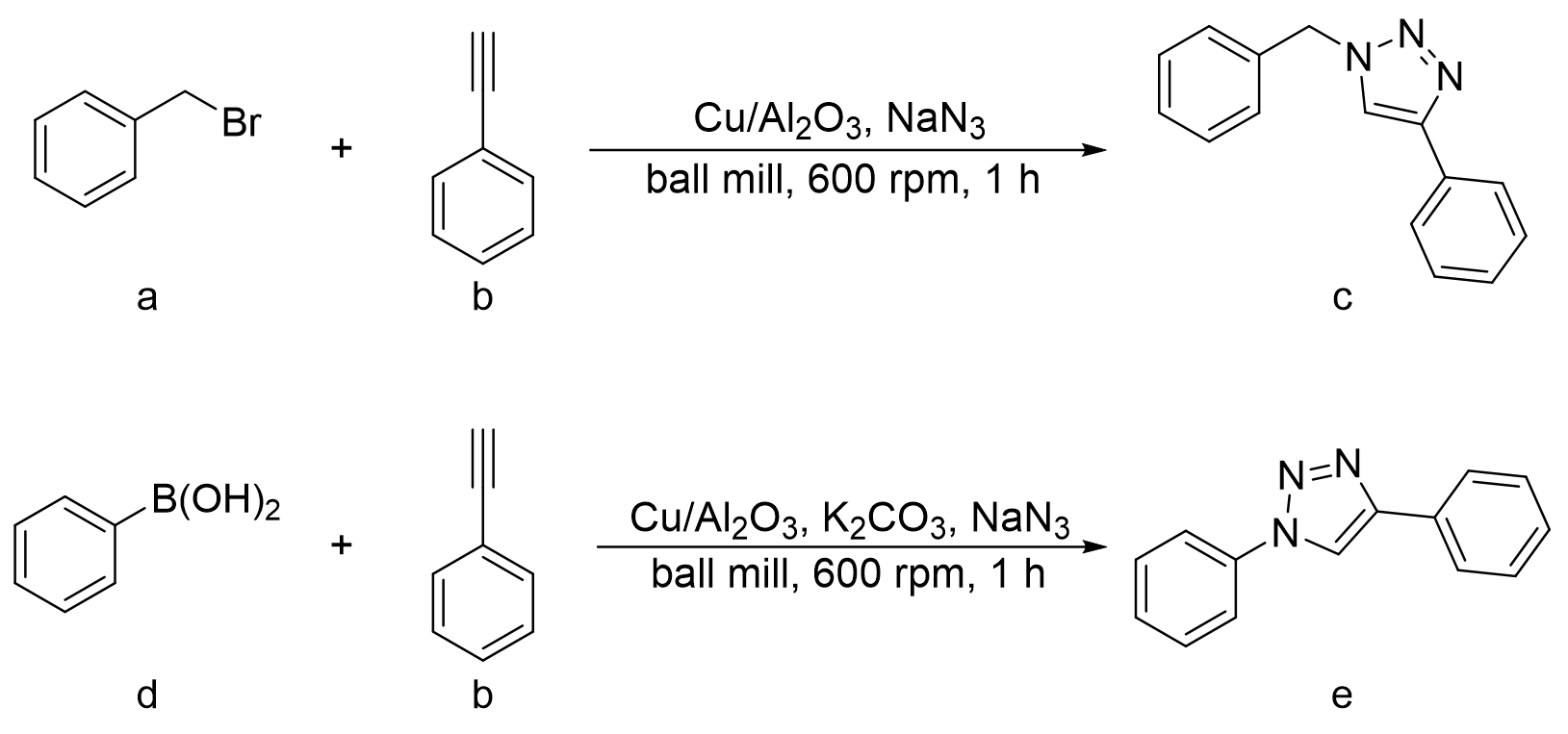 | ||
|---|---|---|
| Number | Time (min) | Yields (%) |
| 1 | 30 | 62 |
| 2 | 45 | 80 |
| 3 | 60 | 96 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
(谷威, 李志强, 朱申敏, 张荻. 化学学报, 2008, (09): 1097.
|
| [5] |
(张修超, 蔡晓兰, 周蕾, 乔颖博, 吴灿, 张爽, 朱伟. 材料导报, 2018, 32(15): 2653.)
|
| [6] |
(张宝剑, 林少芬, 戴乐阳, 刘志杰. 材料导报, 2014, 28(S1): 403.)
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
(许维维. 昆明理工大学硕士论文, 2017.)
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
/
| 〈 |
|
〉 |