

Functionalization and Application of Polymer-Modified Proteins
Received date: 2023-07-10
Revised date: 2023-10-18
Online published: 2024-02-23
Supported by
National Natural Science Foundation of China(22275062)
National Natural Science Foundation of China(22005110)
Undergraduates Research Training Program(S202310487273)
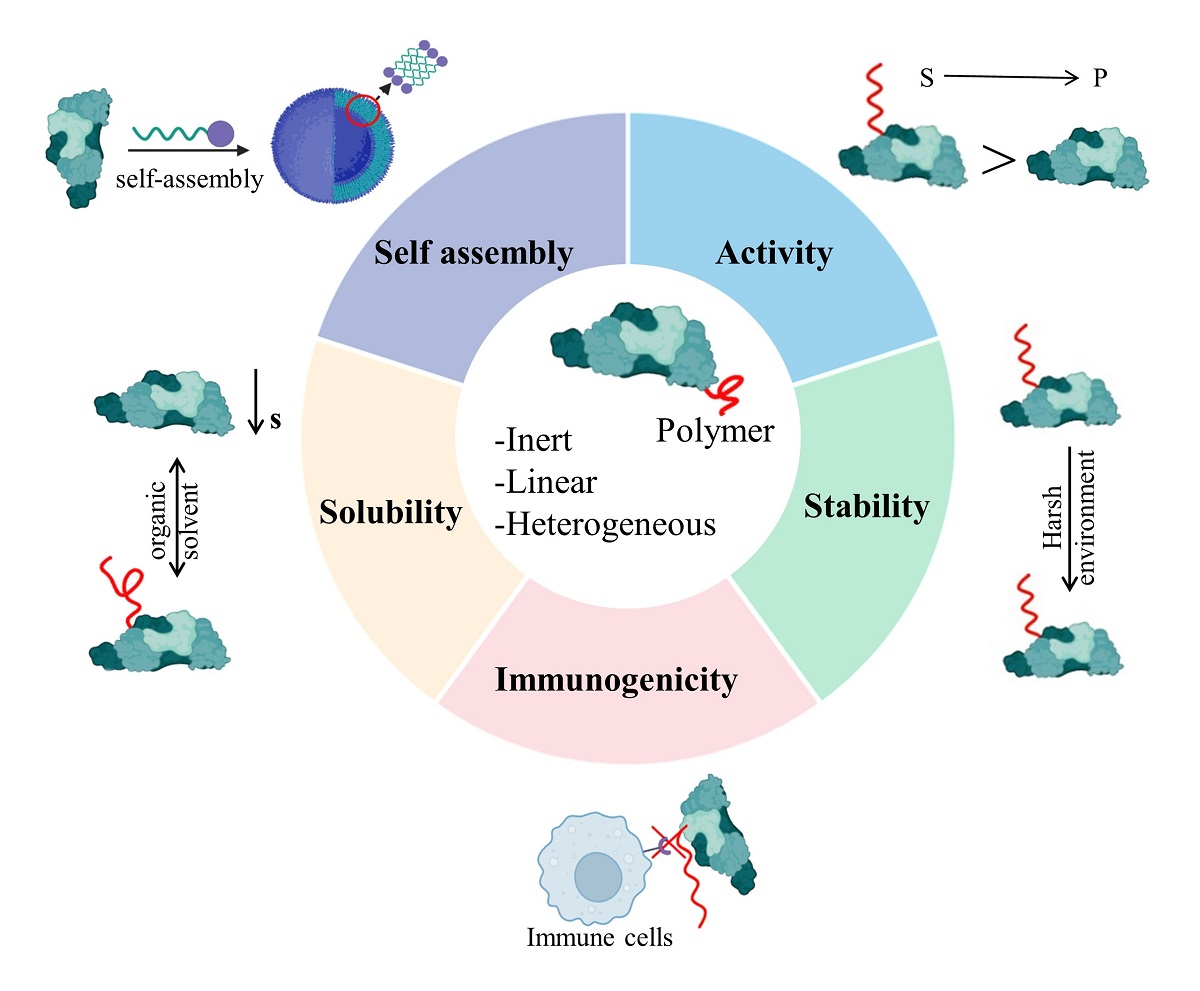
As a kind of important biological macromolecules, proteins have been widely used in chemical and medical fields, such as biocatalysis, drug delivery, and molecular imaging due to their special three-dimensional spatial structure and high catalytic activity. However, there are a series of problems in the practical application of proteins. For example, proteins are easily inactivated in extreme environments. Protein drugs have strong immunogenicity in vivo, which leads to short half-life of drugs and causes adverse reactions in patients easily. Their low solubility in organic solvents limits their use in organic solvents. In order to solve the above problems, researchers have developed methods such as protein engineering and co-immobilization, but there are corresponding shortcomings. Polymer modification is one of the important methods, which can improve the properties of proteins from many aspects and expand the application of proteins. From this point of view, this review focuses on the latest research and classical literature on polymer-modified proteins, and introduces their ingenious modification methods to synthesize materials with excellent properties. The principle, practical application, existing problems and solutions of improving protein stability and activity, immunogenicity, solubility and self-assembly by polymer modification are summarized. On this basis, the challenges and possible development trends in the commercial and clinical translation of this strategy are analyzed.
1 Introduction
2 Stability and activity
2.1 Stability to temperature and pH
2.2 Stability to protease hydrolysis
2.3 Stability of chemical denaturants
2.4 Enhanced enzyme activity
2.5 Regulation of enzyme activity
3 Immunogenicity
4 Solubility
5 Self-assembly
5.1 Drug delivery
5.2 Molecular imaging
6 Conclusion and outlook
Jiang Wan , Jingze Zhang , Hongling Chen , Hanmei Shen , Zhen Wang , Chun Zhang . Functionalization and Application of Polymer-Modified Proteins[J]. Progress in Chemistry, 2024 , 36(3) : 416 -429 . DOI: 10.7536/PC230706
表1 目前正在使用和开发的PEG替代品的例子,PG,聚甘油; PAOx,聚(2-烷基-2- |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
Pelegri-O'Day E M, Lin E W, Maynard H D. J. Am. Chem. Soc., 2014, 136 (41): 14323.
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
Sheremet'ev S V, Lonshakov D V, Belosludtseva E M, Borisova O V, Sidorova A V, Kalinskii A V. Pharm. Chem. J., 2021, 55 (7): 698.
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
(胡玲, 张裕英, 高长有. 化学进展, 2009, 21: 1254.)
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
(李子程, 李攻科, 胡玉玲. 化学进展, 2017, 29: 1480.)
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
(李欢, 杨玉莹, 李国攀, 王席, 胡基雄, 荣明轩, 荣俊. 中国生物制品学杂志, 2021, 34: 941.).
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
/
| 〈 |
|
〉 |