

Applications of Graphene in Hydrogen Evolution Electrocatalyst
Received date: 2023-09-07
Revised date: 2023-11-02
Online published: 2024-04-15
Developing hydrogen energy is an important direction in the future.Industrialized scale electrolysis of water for hydrogen production requires the use of low-cost hydrogen evolution electrocatalyst materials to reduce its overpotential.graphene has shown broad application prospects in hydrogen evolution electrocatalyst materials due to its large specific surface area,excellent conductivity,good stability,adjustable electronic structure,and easy modification of structure and surface state.This article provides a detailed analysis of the mechanism of graphene application in hydrogen evolution electrocatalysis.based on different mechanisms,graphene-based hydrogen evolution electrocatalyst materials were classified and their latest research progress was reviewed.Finally,the future development direction of graphene-based hydrogen evolution electrocatalytic materials was prospected。
1 Introduction
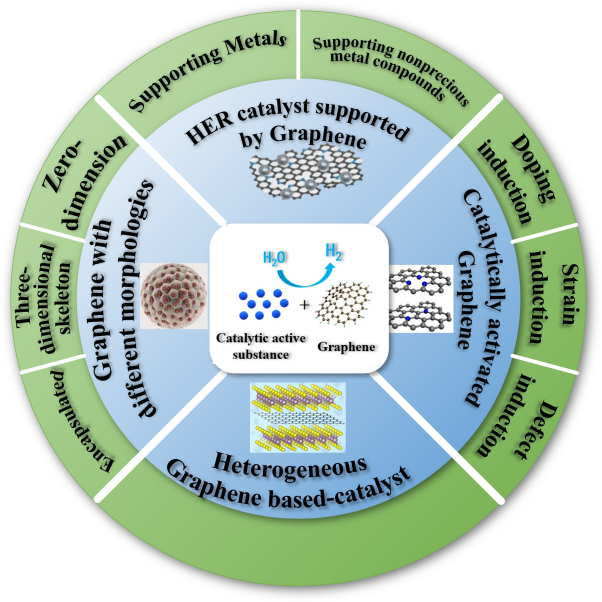
2 HER electrocatalyst supported by Graphene
2.1 Supporting Metals
2.2 Supporting nonprecious metal compounds
3 Catalytically activated-graphene based HER electrocatalyst
3.1 Doping-induced electrocatalytic activity
3.2 Strain-induced electrocatalytic activity
3.3 Defect-induced electrocatalytic activity
4 Heterogeneous graphene-based HER electrocatalyst
5 Graphene with different morphologies based-HER electrocatalyst
5.1 Zero-dimensional graphene
5.2 Three-dimensional skeleton graphene
5.3 Encapsulated graphene
6 Conclusion and outlook
Key words: graphene; hydrogen evolution reaction; electrocatalyst
Yiming Zhang , Jianping Guo , Jiale Zhang , Aowen Zheng , Yanyan Wang , Guangke Tian . Applications of Graphene in Hydrogen Evolution Electrocatalyst[J]. Progress in Chemistry, 2024 , 36(5) : 633 -644 . DOI: 10.7536/PC230905
图2 (A)Pt-CNSs/rGO纳米复合材料的形成机理示意图;(B)Pt-CNSs的透射电子显微镜(TEM)照片,插图:(a)Pt-CNSs的高分辨透射电子显微镜照片(HRTEM)和(b)傅里叶变换(FFT)图;(C)Pt-CNSs/rGO纳米复合物的TEM照片;Pt-CNSs/rGO纳米复合物(D)和Pt-CNSs(E)在充满N2的 0.5 M H2SO4中以 5 mV/s的扫描速率转速为1000 r/min的HER极化曲线和电化学阻抗谱;(D)中插图为Tafel曲线[17]Fig. 2 (A) Schematic illustration of the proposed mechanism for the formation of Pt-CNSs/rGO nanohybrids. (B) TEM image of Pt-CNSs, insets: (a) HRTEM image and (b) FFT pattern of Pt-CNSs. (C) Typical TEM images of Pt-CNSs/rGO nanohybrids. (D) HER polarization curves of Pt-CNSs/rGO nanohybrids and Pt-CNSs in N2-saturated 0.5 M H2SO4 solution at a scan rate of 5 mV/s and rotation rate of 1000 r/min. The top-right inset shows the corresponding Tafel plots for Pt-CNSs/rGO nanohybrids and Pt-CNSs. (E) Electrochemical impedance spectra of Pt-CNSs/rGO nanohybrids and Pt-CNSs[17] |
图3 (A) 在含有石墨烯片的溶液中合成MoS2/GO的示意图;(B)及插图分别是MoS2/rGO复合物的扫描电子显微镜照片(SEM)和TEM照片;(C)在不含石墨烯片的溶液中合成MoS2的示意图;(D)及插图MoS2纳米颗粒的SEM照片和TEM照片;(E)及插图TEM图像显示了复合物中rGO基体上的MoS2颗粒的褶皱边缘;(F)HRTEM图像显示具有高度暴露的边缘的纳米尺寸MoS2堆叠在rGO片上;几种催化剂的极化曲线(G)和Tafel图(H)[26]Fig. 3 (A) Schematic solvothermal synthesis with GO sheets to afford the MoS2/rGO hybrid. (B) SEM and (inset) TEM images of the MoS2/RGO hybrid. (C) Schematic solvothermal synthesis without any GO sheets, resulting in large, free MoS2 particles. (D) SEM and (inset) TEM images of the free particles. (E) TEM image showing folded edges of MoS2 particles on RGO in the hybrid. The inset shows a magnified image of the folded edge of a MoS2 nanoparticle. (F) HRTEM image showing nanosized MoS2 with highly exposed edges stacked on a RGO sheet. Polarization curves (G) and corresponding Tafel plots (H) of different electrocatalysts[26] |
图4 N、S共掺杂石墨烯(NSG)(a)和等离子体刻蚀的N, S共掺杂石墨烯(P-NSG)(b)的低倍TEM图像;NSG(c)和P-NSG(d)的高放大倍率TEM图像;NSG(e)和P-NSG(f)的SAED图;(g)P-NSG合成过程示意图[44]Fig. 4 Low magnification TEM images of N, S co-doped graphene (NSG) (a) and plasma-etched N, S co-doped graphene (P-NSG) (b); high magnification TEM images of NSG (c) and P-NSG (d); SAED patterns of NSG (e) and P-NSG (f); (g) schematic illustration of the synthesis process of P-NSG[44] |
图5 (a) CoFeP和(b) CoFeP/rGO的SEM照片;CoFeP/rGO的TEM(c),HAADF-STEM(d)和成分面扫描图(e~i);CoFeP和CoFeP/rGO在0.5 M H2SO4溶液中的HER性能:(j)HER极化曲线;(k)过电位柱状图;(l)Tafel斜率;(m)电容电流密度;(n)奈奎斯特图;(o)CoFeP/rGO在0.076 V电势下的电流i-时间t曲线[67]Fig. 5 SEM images of (a) CoFeP and (b) CoFeP/rGO, (d) TEM image and corresponding elemental mapping images (e~i) of CoFeP/rGO. HER performance of CoFeP and CoFeP/rGO composites in the 0.5 M H2SO4 solution: (j) HER polarization curves; (k) Overpotentials; (l) Tafel plots; (m) Capacitive current densities; (n) Nyquist plots; (o) i-t curves of CoFeP/rGO at potential of 0.076 V vs RHE[67] |
图6 CoP/G⁞GQD、CoP/G和商业Pt/C的线性伏安(LSV)曲线(A)和Tafel图(B);(C)CoP/G⁞GQD和CoP/G在200 mV过电位下在106到1 Hz的频率范围内测量的奈奎斯特图;(D)CoP/G⁞GQD在-0.17~+0.01 V之间2 mV/s的扫描速率下,经过100 mV/s的2000个CV循环之前和之后的LSV曲线,插图:在91.3 mV的过电位下CoP/G的稳定性测试;(E)CoP/G⁞GQD的合成过程示意图[77]Fig. 6 LSV curves (A) and Tafel plots (B) of CoP/G⁞GQD, CoP/G, and commercial Pt/C; (C) Nyquist plots of CoP/G⁞GQD and CoP/G measured at an overpotential of 200 mV in a frequency range from 106 to 1 Hz; (D) LSV curves of CoP/G⁞GQD at a scan rate of 2 mV/s before and after 2000 CV cycles at a scan rate of 100 mV/s between -0.17 and +0.01 V. Inset: time dependence of the current density of CoP/ G⁞GQD at an overpotential of 91.3 mV; (E) schematic illustration of the synthesis process of CoP/G⁞GQD[77] |
图7 三维MX/CN/RGO纳米结构的形态、微观结构和HER性质:(a)和(b)SEM照片,(c)和(d)TEM照片,(e)高角度环形暗场扫描透射电子显微镜(HAADF-STEM)照片揭示了Ti3C2Tx、g-C3N4纳米片和石墨烯成功集成到3D互穿框架中;(f,g)HR-TEM晶格条纹像;(h)LSV和(i)Tafel 曲线 [82]Fig. 7 Morphological and microstructural analysisof the 3D MX/CN/RGO nanoarchitecture. Representative (a, b) FE-SEM, (c, d) TEM, (e) HAADF-STEM images reveal the successful integration of Ti3C2Tx, g-C3N4 nanosheets and graphene into a 3D interconnected framework; (f, g) HR-TEM images disclose the lattice fringes of Ti3C2Tx and g-C3N4 nanosheets; (h) LSV polarization curves and (i) the corresponding Tafel plots[82] |
图8 石墨烯包裹的CoNi纳米颗粒(CoNi@NC)的HRTEM照片(a,b)以及结构示意图(c);(d)包裹金属纳米颗粒的石墨烯的层数统计柱状图;(e~h)HAADF-STEM图像和成分面分布图[83];(i)各种催化剂上的氢吸附自由能(ΔG(H*));(j)CoNi团簇、CoNi@C和N掺杂石墨烯壳(Ncarbon)中极化电流(i0)与ΔG(H*)之间关系的火山图[83]Fig. 8 (a, b) HRTEM images of CoNi@NC, showing the graphene shells and encapsulated metal nanoparticles. (c) Schematic illustration of the CoNi@NC structure. (d) Statistical analysis of the number of layers in the graphene shells encapsulating the metal nanoparticles in CoNi@NC. (e~h) HAADF-STEM image and corresponding elemental mapping images of CoNi@NC. (i) Gibbs free energy (ΔG) profile of the HER on various catalysts. (j) Volcano plot of the polarized current (i0) versus ΔG(H*) for a CoNi cluster, CoNi@C, and an N-doped graphene shell (Ncarbon)[83] |
表1 Summary of hydrogen evolution electrocatalytic properties of graphene-based electrocatalyst materialsTable 1 Summary of the HER performance of some graphene-based electrocatalysts |
| Type | Material | Electrolyte | Initial overpotential(mV) | 10 mA/cm2 overpotential (mV) | Tafel slope (mV/dec) | Ref | |
|---|---|---|---|---|---|---|---|
| HER electrocatalyst supported by graphene | Supporting metals | Disperse the cobalt onto nitrogen-doped graphene | 0.5 M H2SO4 | 30 | 147 | 82 | 51 |
| Single-atom Ni catalysts anchored to nanoporous graphene | 0.5 M H2SO4 | 50 | — | 45 | 24 | ||
| Mo2TiC2 MXene nanosheets with Ni single atoms loaded on the Mo vacancy sites | 0.5 M H2SO4 | — | 78 | 56.7 | 85 | ||
| MoS2/graphene composite catalyst | 0.5 M H2SO4 | 100 | 183 | 43.3 | 86 | ||
| MoS2+graphene mixture | 201 | 365 | 57.5 | ||||
| Pure MoS2 | 293 | >400 | 114.4 | ||||
| Supporting nonprecious metal compounds | Vertical MoS2 nanosheets on graphene | 0.5 M H2SO4 | 188 | — | 84 | 27 | |
| MoSe2/rGO hybrid nanostructures | 0.5 M H2SO4 | 125 | 195 | 67 | 33 | ||
| MoSe2 | 223 | 390 | 103 | ||||
| N,S co-doped carbon dots intercalated few-layer MoS2/graphene nanosheets | 0.5 M H2SO4 | 37 | 98 | 53 | 87 | ||
| Catalytically activated-graphene based HER electrocatalyst | Doping-induced electrocatalytic activity | S,N-doped graphene | 0.5 M H2SO4 | — | 280 | 80.5 | 10 |
| B-substituted graphene | 0.5 M H2SO4 | 200 | 440 | 99 | 47 | ||
| Defective graphene | 300 | — | 130 | ||||
| N-doped mesoporous graphene | 0.5 M H2SO4 | — | 239 | 109 | 48 | ||
| Ni heterolayer N-doped graphene composite MoS2 | 0.5 M H2SO4 | 60 | 270 | 56 | 57 | ||
| Nickel heterolayer MoS2 | 285 | 460 | 78 | ||||
| Graphene based six membered C-ring dual N-doping | 0.5 M H2SO4 | — | 57 | 44.6 | 58 | ||
| Ultrafine cobalt-ruthenium alloy on nitrogen and phosphorus co-doped graphene | 0.5 M H2SO4 | — | 52 | 38 | 88 | ||
| Strain-induced electrocatalytic activity | Mechanical strain and interfacial-chemical interaction for 1T Co-doped WSe2/carbon nanotubes | 0.5 M H2SO4 | — | 147 | 33 | 89 | |
| Tuning surface lattice strain towards CoPt2/C truncated octahedron | 0.5 M H2SO4 | — | 17 | 35 | 90 | ||
| Defect-induced electrocatalytic activity | Double defect N-doped graphene | 0.5 M H2SO4 | — | 245 | 141 | 91 | |
| Single atom S vacancy defect WS2 nanosheets | 0.5 M H2SO4 | — | 137 | 53.9 | 92 | ||
| Single atom S vacancy defect WS2 nanosheets loaded on defective graphene | — | 108 | 48.3 | ||||
| Heterogeneous graphene-based HER electrocatalyst | CoFeP/graphene heterostructure | 0.5 M H2SO4 | — | 76 | — | 67 | |
| graphene /CoMo3S13 sulfur gel heterostructure | 0.5 M H2SO4 | — | 130 | 40.1 | 68 | ||
| MoS2/ graphene heterostructure | 0.5 M H2SO4 | — | 120 | 72 | 69 | ||
| Graphene with different morphologies based-HER electrocatalyst | Zero-dimensional graphene | Coral-shaped MoS2 decorated with graphene quantum dots | 0.5 M H2SO4 | 95 | 120 | 40 | 75 |
| Coral-shaped MoS2 | 124 | 173 | 63 | ||||
| Synthesis of CoP nanoparticles supported on pristine graphene by graphene quantum dots | 0.5 M H2SO4 | 7 | 91.3 | 42.6 | 77 | ||
| CoP nanoparticles supported on pristine graphene | 118.9 | 156.89 | 70.22 | ||||
| graphene quantum dots /MoS2 microsheets | 0.5 M H2SO4 | — | 160 | 56.9 | 93 | ||
| MoS2 microsheets | — | 340 | 93.6 | ||||
| Ultrafine graphene like C3N4 quantum dots | 0.5 M H2SO4 | — | 208 | 52 | 94 | ||
| Three-dimensional skeleton graphene | loading of vertical graphene sheets on SiOx nanowires | 0.5 M H2SO4 | 18 | 107 | 64 | 78 | |
| 3D interweaved MXene/graphitic carbon nitride nanosheets/graphene nanoarchitectures | 0.5 M H2SO4 | 38 | — | 76 | 82 | ||
| Three-dimensional foliated MoS2/rGO composite aerogel | 0.5 M H2SO4 | 105 | — | 51 | 95 | ||
| MoS2 | 216 | — | 89 | ||||
| Encapsulated graphene | Ultrathin graphene shell encapsulated CoNi nanoalloy | 0.5 M H2SO4 | Almost 0 | 142 | 104 | 83 | |
| N-doped graphene encapsulated Ni3Cu1 nanoflower | 0.5 M H2SO4 | — | 95 | 77.1 | 84 | ||
| N-doped carbon encapsulated CoP nanoparticles | 0.5 M H2SO4 | — | 135 | 59.3 | 96 | ||
| CoP | — | 231 | 85.8 | ||||
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
/
| 〈 |
|
〉 |