

Application of New Separation and Purification Technology in Natural Products
Received date: 2023-08-15
Revised date: 2023-12-20
Online published: 2024-03-15
Supported by
National Key R&D Program of China(2019YFA0904104)
Scientific Research Cooperation Program(202221641061A)
Scientific Research Cooperation Program(202321641067A)
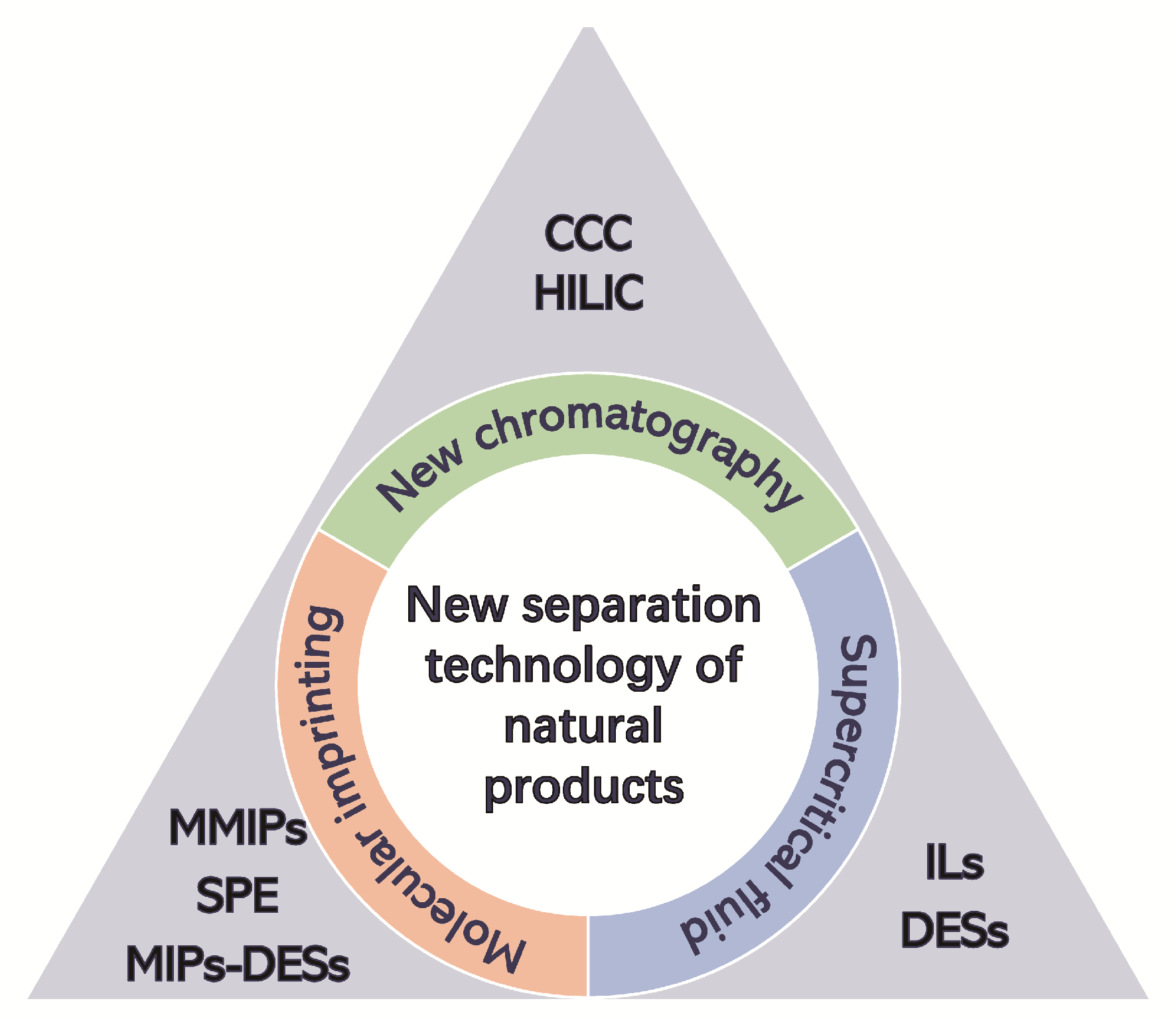
natural products are secondary metabolites preserved by natural selection in the long-term evolution process of natural organisms,and are widely used in many fields because of their rich medicinal value.With the development of modern science,the demand for high purity products of natural products is also increasing.Traditional separation methods usually have some disadvantages such as large consumption of organic solvents,poor separation effect,high cost and long cycle,which seriously restrict the development and use of natural products in various fields.the emergence of new separation and purification technology provides a new idea for the extraction,separation and application of natural products.On the basis of summarizing the existing literature,this paper reviews the new methods of separation and purification of natural products,and finally summarizes and discusses the research bottleneck and future development direction of natural product separation and purification。
1 Introduction
2 Novel chromatographic method
2.1 Counter-current chromatography
2.2 Hydrophilic interaction chromatography
3 Supercritical fluid separation
3.1 Ionic liquid separation
3.2 Deep eutectic solvent separation
3.3 Ionic liquid and deep eutectic solvent cooperation
4 Molecular imprinting technology
4.1 Magnetic molecularly imprinted polymer
4.2 Molecular imprinted solid phase extraction
4.3 Deep eutectic solvent-molecularly imprinted polymer
5 Conclusion and outlook
Key words: natural products; separation; purification
Wenwei Li , Ziyu Zhu , Ruilin Haotian , Yao Xie , Aiqin Luo , Axin Liang . Application of New Separation and Purification Technology in Natural Products[J]. Progress in Chemistry, 2024 , 36(5) : 667 -678 . DOI: 10.7536/PC230809
表1 Application of Supercritical Fluid in Separation of Natural ProductsTable 1 Application of supercritical fluids in the separation of natural products |
| Target | Source | Phase component | Separation condition | Extraction yield | Ref |
|---|---|---|---|---|---|
| Flavonoids | Eucommia ulmoides leaves | [Ch][Try] | IL 2.91%,73℃ | 78.0% | 73 |
| Ginkgo biloba leaves | Menthol-acetic acid DES micellar system | IL 0.2 g/mL,25℃ | 97.29% | 74 | |
| M. oleifera leaves | [ChCl][CA](1:1) | 20 mL DES was added to crushed leaves at a ratio of 1:10 (m/v). The mixture was homogenized in a sonicator bath at 50℃ for 1 h. | - | 75 | |
| Alkaloids | Scutellaria baicalensis Georgi | [C2min]Cl, [C4min]Cl, KPF6 | 60℃ for 1 h, ultrasound-assisted extraction for 5 min | 43.3 mg/g | 76 |
| Berberidis Radix | [ChCL][LA](1:2) | 200 W ultrasonic irradiation at 50℃ for 30 min | 75.00% | 77 | |
| Aqueous solution | [C2mim]Cl/PEG 2000 | 50℃, atmospheric pressure | 70.70% | 78 | |
| Terpenoids | Licorice | [P4,4,4][PTS] | Less than 60℃ for 30 min | 9.63 mg/g | 79 |
| Panax notoginseng | ChCl-ethylene glycol DES/K2HPO4 | 55℃, pH=5.0 | 75.79% | 80 | |
| Polyphenolics | Cistanche tubulosa | [C4mim]BF4/(NH4)2SO4 | sample solution of 2.5 mg/mL, pH=6.0; 130 min | 99.78% | 81 |
表2 Application of molecular imprinting technique in natural product separation in recent yearsTable 2 Application of molecular imprinting technique in separation of natural products in recent years |
| Template molecule | Functional monomers | Cross-linkers | Extraction yield | Ref | |
|---|---|---|---|---|---|
| Magnetic molecularly imprinted polymer | Chlorogenic acid | Acrylamide (AM) | Ethylene glycol dimethacrylate (EGDMA) | 3.86 μg/mL | 87 |
| Chrysin | Methacrylic acid (MAA) | EGDMA | 35.27 mg/g | 99 | |
| Ferulic acid | MAA | Polyvinylpolypyrrolidone (PVPP) | 50 mg/g | 100 | |
| Rutin | 4-vinylpyridine(4-VP), 4-vinylphenylboronic acid (4-VPBA) | Divinylbenzene (DVB) | 11.9 mg/g | 89 | |
| Molecular imprinted solid phase extraction | Chlorogenic acid | MAA, AM, 2-VP | EGDMA | 75.15% | 94 |
| (E)-resveratrol | 4-VP | EGDMA | 96% | 101 | |
| Harmaline | MAA | Ethylene glycol dimethacrylate (EDMA) | 45.31 mg/g | 102 | |
| Fsesquiterpene coumarins | MAA, methacrylamide (MAAM), 4-VP | EDGMA | 0.8 μg/g | 103 | |
| DESs-MIP | Quercetagetin | 2-VP | EGDMA | 27.2 mg/g | 98 |
| Catechins | DESs were formed from ChCl/MAA (molar ratio 1:2) and betaine/MAA/H2O (molar ratio 1:2:1) | EDMA | 13.1 mg/g | 104 | |
| Paclitaxel | DESs | EGDMA | 87.08 mg/g | 105 | |
| Gallic Acid | DES | EGDMA | 87.85 % | 106 |
表3 Comparison of Different Separation Methods for Natural ProductsTable 3 Comparison of different natural products separation methods |
| Techique | Principles | Advantages | Disadvantages | Range of application | Ref |
|---|---|---|---|---|---|
| Fractional precipitation | Like dissolves like | Simplicity of operator, solvent environmental protection | Poor separation, need to be repeated several times. | Substances of very different polarity | 18 |
| Gel chromatography | Different molecular sizes | High purification, high separation efficiency, mild conditions | High cost, complex conditions | Substances with different molecular weights | 19 |
| Cellulose column chromatography (DEAE) | Eluted in descending order of water solubility. | Good purification effect, large amount of separation | Complex operation, slow separation | Substances with different molecular weights | 20 |
| Preparative high Performance liquid chromatography | The difference in the distribution coefficient. | High purification, high precision, high stability, good reproducibility | High cost, complex process, long time | Substances of different polarity | 21 |
| Separation membrane | Different molecular sizes. | Little damage to polysaccharide activity, good separation effect | Poor separation effect for substances with similar molecular weight. | Substances with different molecular weights | 22 |
| Macroporous resin | The difference of adsorption force and molecular weight. | Good selectivity, easy regeneration treatment, mild desorption conditions, long service life | Pretreatment requirements are strict | Substances of different polarity | 23 |
| High-speed Counter-current Chromatography (HSCCC) | The difference in the distribution coefficient. | Small sample loss, large amount of preparation, can separate structurally similar substances | Low separation efficiency, large solvent consumption | Suitable for separation of polar substances | 26~27,40⇓⇓⇓⇓⇓ ~46 |
| Hydrophilic interaction chromatography(HILIC) | Uses the charge and hydrophilicity on the molecular surface to control the separation of molecules. | Good ability to separate polar compounds, separation without affecting sample properties | Large influence of parameters | For the simultaneous separation of both polar and small polar compounds | 28~29,47⇓⇓⇓⇓ ~52 |
| Ionic liquids(IL)/ Deep eutectic solvent (DES) | Design molecular structures to separate different substances. | Eco-Friendly, good solubility, high electrical conductivity, low toxicity | Hard to be separated from the sample, high cost | Liquid mixture | 30~31,53⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓ ~81 |
| Molecular imprinting technique (MIT) | “Antigen-antibody” specific binding | Strong specificity, long service life, good stability, low cost | The selectivity is poor in aqueous solution. | Mainly in the field of small molecules | 32⇓~34,82⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓ ~106 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
( 张晓娇, 孙立权, 罗爱芹, 杨学东, 张蕊. 食品工业科技, 2019, 31(7): 996.)
|
| [36] |
( 孙立权, 杨学东, 孙琛瑜, 汤波, 罗爱芹. 北京理工大学学报, 2019, 39(11): 1207.)
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
Veeraraghavan Ramachandran P,
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
/
| 〈 |
|
〉 |