

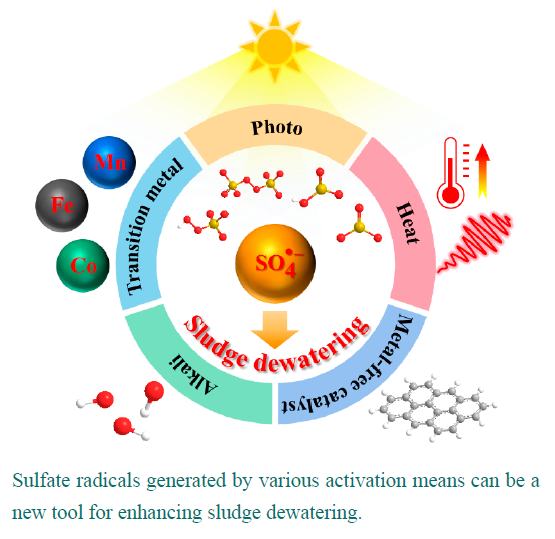
Sulfate Radicals: A New Tool for Enhancing Sludge Dewatering
Received date: 2023-07-14
Revised date: 2024-01-28
Online published: 2024-04-16
Supported by
Open Fund of Key Laboratory of Green Technology in Ecological Industry of Fujian Province(WYKF-EIGT2021-3)
sludge is an inevitable by-product of the wastewater treatment process and due to its high water content,large volume,and inclusion of a large amount of toxic and hazardous substances,needs to be minimized and harmlessly treated.However,sludge possesses extracellular polymeric substances(EPS)formed by ionization of negatively charged functional groups,which maintain a stable hydrated colloidal structure and prevent the release of water.this is a key factor in the difficulty of sludge dewatering.in the last decade,sulfate radical-Based advanced oxidation processes(SR-AOPs)have received considerable attention due to their high efficiency for EPS disintegration,rapid reaction time and environmental friendliness,and thus sulfate radicals have become a new powerful tool for enhancing sludge dewaterability.in This paper,the development timeline and activation mechanisms of sulfate radicals are reviewed in detail,and the research advances of SR-AOPs for improving sludge dewaterability and removing micropollutants and heavy metals from sludge are systematically evaluated.based on the current scientific problems of SR-AOPs in sludge conditioning,the future research directions of SR-AOPs are proposed from the perspectives of mechanism research,cost-effectiveness,and experimental scale,in order to provide a solid theoretical reference for sludge conditioning in wastewater treatment plants in China。
1 Introduction
2 Timeline of sulfate radicals
3 Mechanism of sulfate radicals activation
3.1 Transition metal activation
3.2 Photo activation
3.3 Heat activation
3.4 Alkali activation
3.5 Metal-free catalyst activation
4 Sludge dewaterability improved by SR-AOPs
4.1 Iron activated methods
4.2 Heat activated methods
4.3 Electrochemically activated methods
4.4 Alkali activated methods
4.5 Other activation methods
5 Removal of micropollutants
6 Removal of heavy metals
7 Sludge dewatering mechanism
8 Conclusion and outlook
Yue Lai , Chao Wang , Jie Zhang , Shungui Zhou , Changgeng Liu , Jie Ye . Sulfate Radicals: A New Tool for Enhancing Sludge Dewatering[J]. Progress in Chemistry, 2024 , 36(5) : 798 -814 . DOI: 10.7536/PC230707
表1 Mechanism of activation of different precursors to produce sulfate radicalTable 1 Activation of different precursors to produce sulfate radical mechanisms |
| Activation method | Precursor | Mechanism | Predominant radical species | Comments | Ref |
|---|---|---|---|---|---|
| Transition metals | PMS | One electron transfer | SO4 | / | 62 |
| PDS | One electron transfer | SO4 | / | 62 | |
| SF | Involves several transient short-lived oxysulfur radicals initiated by direct one-electron transfer from SF to metal ions | $\mathrm{SO}_{4}^{\centerdot -}$/$\cdot \ \mathrm{OH}$ | / | 63 | |
| Photo | PMS | Breaking of O−O bond | $\mathrm{SO}_{4}^{\centerdot -}$/$\cdot \ \mathrm{OH}$ | / | 64 |
| PDS | Breaking of O−O bond | SO4 | / | 65 | |
| SF | Photoelectrons and holes can be produced from photocatalysts, the generated holes can activate sulfite to form oxysulfur radicals | $\mathrm{SO}_{4}^{\centerdot -}$/$\cdot \ \mathrm{OH}$/$\mathrm{O}_{2}^{\centerdot -}$ | / | 15 | |
| Heat | PMS | Breaking of O−O bond | $\mathrm{SO}_{4}^{\centerdot -}$/$\cdot \ \mathrm{OH}$ | The bond dissociation energy is higher, so a higher temperature is required to cleave O−O bond | 66 |
| PDS | Breaking of O−O bond | $\mathrm{SO}_{4}^{\centerdot -}$/$\cdot \ \mathrm{OH}$ | The bond dissociation energy is lower, and increasing the temperature can effectively cleave O−O bond | 67 | |
| SF | SF autoxidation generates oxygen-sulfur radicals | $\mathrm{SO}_{4}^{\centerdot -}$/$\cdot \ \mathrm{OH}$ | / | 68 | |
| Alkali | PMS | Alkali-catalyzed hydrolysis of PMS to hydrogen peroxide | O2 | / | 69 |
| PDS | Alkali-catalyzed hydrolysis of PDS to hydroperoxides triggers free radical formation | $\mathrm{SO}_{4}^{\centerdot -}$/$\cdot \ \mathrm{OH}$/$\mathrm{O}_{2}^{\centerdot -}$ | pH > 11 | 70 | |
| SF | / | / | / | / | |
| Metal-free catalyst | PMS | One electron transfer | SO4 | Graphene exhibits better catalytic properties than several other carbon isomers, including: activated carbon, graphite, graphene oxide, and carbon nanotubes | 38 |
| PDS | Peroxide bond of PDS is weakened at the defective edge of the carbon catalyst and oxygen groups (of which the carbonyl group is the most active) | OH | Reduced mesoporous carbon, carbon nanotubes, and graphene oxide, displayed great catalytic properties, in contrary to nanodiamonds, fullerenes and graphitic carbon nitride | 39 | |
| SF | Bonding to carbon catalyst ketone groups to form complexes with internal electron transfer leading to the formation of oxygen-sulphur radicals | SO4 | / | 71 |
表2 Effect of different iron activators on improvement of sludge dewaterabilityTable 2 Effect of different iron activator on the improvement of sludge dewaterability |
| Catalyst | Chemical | Assisted activation method | Solid content | Dosage | Sludge type | CST(s) | CST reduction(%) | Ref |
|---|---|---|---|---|---|---|---|---|
| Fe2+ | PMS | / | TSS = 12.3 g/L | Fe2+ = 0.81 mmol/g VSS PMS = 0.9 mmol/g VSS | WAS | 201.8 | 90 | 87 |
| / | TSS = 20.4 g/L | Fe2+ = 0.81 mmol/g VSS PMS = 0.9 mmol/g VSS | WAS | 49.1 | 45.67 | 86 | ||
| Chelator | TS = 25.6 g/L | Fe2+ = 0.6 mmol/g VS PMS = 0.9 mmol/g VS Chelator = 0.3 mmol/g VS | ADS | 130.8 | 88.3 | 107 | ||
| Chelator | TSS = 51.62 g/L | Fe2+ = 1.2 mmol/g VSS PMS = 1.0 mmol/g VSS Chelator = 0.4 mmol/g VSS | ADS | 599.9 | 60 | 106 | ||
| PDS | / | TSS = 11.9 g/L | Fe2+ = 1.5 mmol/g VSS PDS = 1.2 mmol/g VSS | WAS | 502.4 | 86.4 | 97 | |
| / | TS = 26.2 g/L | Fe2+ = 2.0 mmol/g VS PDS = 1.6 mmol/g VS | WAS | 681.0 | 88.0 | 96 | ||
| / | TSS = 20.7 g/L | Fe2+ = 1.5 mmol/g VSS PDS = 1.2 mmol/g VSS | WAS | 573.4 | 94.2 | 94 | ||
| Chelator | TS = 20.14 g/L | Fe2+ = 0.6 mmol/g TS PDS = 0.4 mmol/g TS Chelator = 0.15 mmol/g TS | WAS | 836.6 | 96.4 | 109 | ||
| Chelator | TS = 19.9 g/L | Fe2+ = 0.3 mmol/g TS PDS = 0.6 mmol/g TS Chelator = 0.15 mmol/g TS | WAS | 720.4 | 61.5 | 83 | ||
| Skeleton | TSS = 35 g/L | Fe2+ = 23.5 mg/g DS PDS = 100 mg/g DS Skeleton = 300 mg/g DS | WAS | 182 | 84.6 | 111 | ||
| SF | / | VSS/TSS = 51.88% | Fe2+ = 1.79 mmol/L TSS SF = 1.43 mmol/L TSS | WAS | 139.7 | 68.9 | 45 | |
| ZVI | PMS | / | TSS = 23.4 g/L | ZVI = 0.25 g/g TSS PMS = 0.1 g/g TSS | WAS | 48.6 | ~50 | 88 |
| PDS | / | TS = 28.5 g/L | ZVI = 2 g/g TS PDS = 0.5 g/g TS | ADS | 174.7 | 90 | 92 | |
| / | TS = 7.9 g/L | ZVI = 15 g/L PDS = 4 g/L | WAS | 20.5 | 50.2 | 84 | ||
| / | TSS = 15.2 g/L | ZVI = 2 g/g TSS PDS = 0.5 g/g TSS | ADS | 144.6 | 42.6 | 89 | ||
| / | TS = 26.1 g/L | ZVI = 0.05 g/g TS PDS = 0.1 g/g TS | ADS | 119.1 | ~80 | 98 | ||
| Chelator | TSS = 56.37 g/L | ZVI = 0.5 mmol/g TSS PDS = 0.5 mmol/g TSS Chelator = 0.05 mmol/g TSS | WAS | 254.9 | 51.7 | 108 | ||
| SF | / | TS = 23.6 g/L | ZVI = 0.9 mmol/g VS SF = 1.2 mmol/g VS | ADS | 120.9 | 81.7 | 55 |
表3 Sludge dewaterability after thermal activated PMS/PDS oxidation conditioningTable 3 Sludge dewatering performance after treatment by thermally activated PMS/PDS oxidation |
| Temperature (℃) | Chemical | Sludge type | Solid content(g/L) | Dosage | Reaction time | CST(s) | CST reduction(%) | Ref |
|---|---|---|---|---|---|---|---|---|
| 120 | PDS | WAS | TSS = 45.21 | ZVI = 100 mg/g TSS PDS = 200 mg/g TSS | 30 min | 190.6 | 72.4 | 122 |
| 80 | PDS | WAS | TSS = 16.03 | PDS = 2.0 mmol/g VSS | 60 min | 88.1 | 76.6 | 121 |
| PMS | PMS = 0.5 mmol/g VSS | 53.9 | ||||||
| 70 | PDS | WAS | TS = 16.5 | PDS = 120 mg/g VS Skeleton = 150 mg/g VS | 10 min | 163.5 | 65.5 | 120 |
| 80 | PDS | WAS | TSS = 41.3 | PDS = 2.0 mmol/g VSS | 60 min | 54.0 | 40.3 | 119 |
| 75 | PMS | WAS | TS = 12.6 | PMS = 150 mg/g VS Skeleton = 100~400 mg/g VS | 40 min | 158.5 | 63.9 | 126 |
| 55 | PDS | ADS | TS = 29.3 | PDS = 3 g/L | 24 h | 230 | 33.2 | 127 |
| PMS | PMS = 3 g/L | 28.0 | ||||||
| 120 | PDS | WAS | TSS = 24.49 | PDS = 120 mg/g TSS | 30 min | 100.7 | 57.6 | 128 |
| 60 | PMS | WAS | TSS = 13.6 | Fe2+ = 0.6 mmol/g VSS PMS = 1.0 mmol/g VSS | 20 min | 206.3 | 92.9 | 124 |
| 60 | PDS | WAS | TSS = 26.4 | Fe2+ = 1.5 mmol/g VSS PDS = 1.2 mmol/g VSS | 20 min | 3006.1 | 96.6 | 123 |
表4 Degradation of trace pollutants in sludge by different SR-AOPsTable 4 Degradation of micropollutant in sludge by different SR-AOPs |
| Item | Process | Chemical | Dosage | Micropollutant | Initial concentration | Removal rate(%) | Ref |
|---|---|---|---|---|---|---|---|
| Aromatic compounds | poly(3-hydroxybutyrate)/PMS Fe2+/S2O82- | PMS PDS | PMS = 3.1 × 10-4 M PHB = 3.3 g/L Fe2+ = 2.0 mmol/g VS PDS = 1.6 mmol/g VS | PAHs | 3099 ± 32 ng/g dw 2692.0 μg/kg | 79 | 149 96 |
| Toluene | 89.8 | ||||||
| Biochar/S2O82- | PDS | PDS = 10 mmol/L Catalyst = 0.5 g/L | Phenol | 200 mg/L | 57.8 | 150 | |
| Phenolic compounds | Biochar/S2O82- | PDS | PDS = 10 mmol/L Catalyst = 0.5 g/L | Phenol | 200 mg/L | 57.8 | 150 |
| Antibiotics | MW(160 ℃)/S2O82- | PDS | PDS = 0.87 g/g TS | Oxytetracycline | 2 mg/g TS | 98.9 | 151 |
| Ciprofloxacin | 98.3 | ||||||
| Modified sludge bio-hydrochar/S2O82- | PDS | PDS = 5 mmol/L Catalyst = 0.2 g/L | Tetracycline | 60 mg/L | 99.72 | 152 | |
| Fe2+/75 ℃/S2O82- | PDS | PDS = 22.7 mM | Oxytetracycline | 100 mg/kg TS | 95 | 125 | |
| Ciprofloxacin | 84 | ||||||
| Triclosan | >99 | ||||||
| Fenton sludge-Cu/PMS | PMS | PMS = 20 mg/L Catalyst = 0.2 g/L | Tetracycline | 10 mg/L | 85.53 | 153 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
(杨秦辉. 哈尔滨工业大学硕士论文, 2019).
|
| [12] |
(肖一帆. 南京农业大学硕士论文, 2019).
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
(刘军. 湖南大学硕士论文, 2017).
|
| [75] |
(薛茂. 哈尔滨工业大学硕士论文, 2019).
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
(李娜. 华中科技大学硕士论文, 2019).
|
| [161] |
|
| [162] |
|
| [163] |
|
/
| 〈 |
|
〉 |