

Multifunctional Organic Luminescent Materials Based on Benzophenone Frameworks
Received date: 2023-03-13
Revised date: 2023-05-12
Online published: 2023-05-18
Supported by
National Natural Science Foundation of China(21574068)
Major Research Program from the State Ministry of Science and Technology,(2012CB933301)
Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD)
Priority Academic Program Development of Jiangsu Higher Education Institutions(YX030003)
Project of State Key Laboratory of Organic Electronics and Information Displays, Nanjing University of Posts and Telecommunication(GZR2023010056)
The optoelectronic properties of organic luminescent materials are strongly correlated with the molecular structure, the flexibility of conformational change and the intermolecular interaction. From the perspective of structure, the carbonyl group and benzene ring of benzophenone have high chemical modifiability. In this paper, the chemical synthesis methods to produce multifunctional organic luminescent materials based on benzophenone framework in recent years are systematically reviewed, including three basic strategies: multiple substitution of benzophenone, using heteroatom as bridging group, vinyl coupling and direct coupling of benzene ring as the center. A variety of multifunctional organic luminescent materials based on this framework have been developed, including fluorescence materials, hosts of precious metal phosphorescence complex, thermally activated delayed fluorescence materials, aggregation-induced emission materials and pure organic room temperature phosphorescence materials. Finally, the development prospect of multi-functional organic luminescent materials based on benzophenone framework is prospected.
1 Introduction
2 Fluorescence materials based on benzophenone framework
3 Hosts based on benzophenone framework for precious metal phosphorescence complex
4 Thermally activated delayed fluorescence materials based on benzophenone framework
5 Aggregation-induced emission materials based on benzophenone framework
6 Pure organic room temperature phosphorescence materials based on benzophenone framework
7 Conclusions and outlook
Wei Tang , Yan Bing , Xudong Liu , Hongji Jiang . Multifunctional Organic Luminescent Materials Based on Benzophenone Frameworks[J]. Progress in Chemistry, 2023 , 35(10) : 1461 -1485 . DOI: 10.7536/PC230306
图1 (a)有机发光材料的发光机理;(b)室温和77 K条件下,二苯甲酮四氢呋喃溶液的光致发光光谱以及室温结晶的光致发光光谱[5]Fig.1 (a) The luminescence mechanism of organic luminescent materials. (b) Photoluminescence spectra of benzophenone in tetrahydrofuran solution at room temperature and 77 K, and photoluminescence spectrum of crystalline state at room temperature[5] |
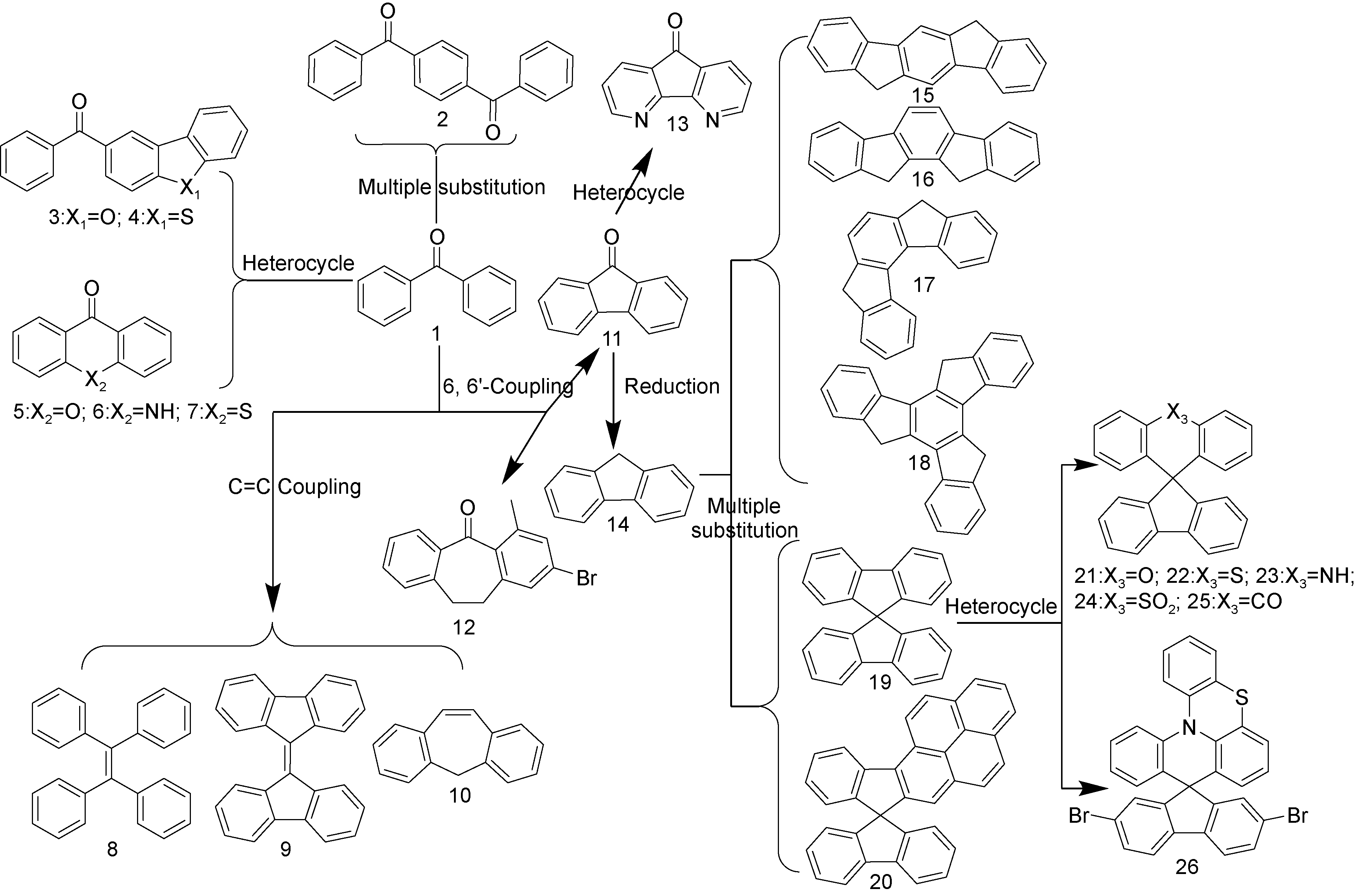
图2 二苯甲酮及其衍生物分子骨架的化学合成策略Fig.2 Chemical modification strategies for the molecular skeletons of benzophenone and their derivatives |
图3 二苯甲酮和多取代二苯甲酮的合成方法Fig.3 Synthesis of benzophenone and polysubstituted benzophenone |
表1 二苯甲酮的光物理和热稳定性参数[5,7⇓⇓~10]Table 1 Optical physics and thermal stability parameters of benzophenone[5,7⇓⇓~10] |
| material | (nm) | (nm) | PLQYc)/d) (%) | τd) (μs) | S1/ (eV) | HOMO/LUMOe) (eV) | Td (oC) | Tg (oC) |
|---|---|---|---|---|---|---|---|---|
| benzophenone | 310/380 | 410/440/472 | 0.001/15.9 | 312.9 | 3.6/2.9 | -4.04/0.56 | 190 | -56 |
a) 室温四氢呋喃(1 μmol/L);b) 77 K四氢呋喃(1 μmol/L);c) 室温乙腈(1 μmol/L);d) 室温晶体;e) 理论计算 |
图5 以C=C偶联和苯环为中心直接偶联的二苯甲酮衍生物的合成方法Fig.5 Synthesis of benzophenone derivatives with vinyl coupling and direct coupling of phenyl ring |
图7 (a)33的化学结构;(b)不同样品电压下33的瞬态光电流(插入显示线性图中的一个瞬态曲线,插图上的箭头表示孔的传输时间);(c)空穴传输材料33在非晶层中空穴漂移迁移率与外加电场的关系[54]Fig.7 (a) Chemical structure of 33. (b) Transient photocurrents in the layers of 33 at different sample voltages. Inserts show the one transient curve in linear plot. Arrows on insets indicate a transit time of holes. (c) The dependences of hole-drift mobility on the applied electric field in the amorphous layers of 33[54] |
图17 (a)52和53的化学结构;(b)52和53激子跃迁路线,图下部为各材料光致发光过程中瞬时荧光和延迟荧光的占比[104]Fig.17 (a) Chemical structures of 52 and 53. (b) Exciton transition routes of 52 and 53. The ratio of transient fluorescence and delayed fluorescence in the photoluminescence process of each material is shown at the bottom of the graph[104] |
图23 (a)68和69的化学结构;(b)EQE、功率效率和电流效率与OLED的亮度特性;(c)薄膜的荧光光谱、不同工作电压下的电致发光谱和掺杂OLED的照片[132]Fig.23 (a) Chemical structures of 68 and 69. (b) EQE, power efficiency and current efficiency versus luminance characteristics of the OLED. (c) PL spectra, EL spectra at various operating voltages and photographs of doped OLED[132] |
图28 (a)81的化学结构;(b)81在不同庚烷组分的CHCl3和庚烷混合物(fw)中的光致发光光谱;(c)相对强度(I/I0)随不同组分氯仿和庚烷混合物的变化。插图显示了在365nm光照下,CHCl3(fw=0,左)和CHCl3和庚烷混合物(fw=90%,右)中的81[154]Fig.28 (a) Chemical structure of 81. (b) Photoluminescence spectra of 81 in CHCl3 and heptane mixtures with different heptane components (fw). (c) The relative strength (I/I0) changes with different components CHCl3 and heptane mixtures (5×10-6 M). The inset shows 81 in CHCl3 (fw=0, left) and CHCl3 and heptane mixtures (fw=90%, right) at 365nm illumination[154] |
图30 (a)83~87的化学结构;(b)83~87在日光(上)和室温365nm紫外灯照明下(下)晶体照片以及87的氮气脱氧环己烷溶液(10-4 mol/L)在365nm紫外灯照射下的未发光(300 K,上)和磷光(77 K,下)照片[5,170,173]Fig.30 (a) Chemical structures of 83~87. (b) Crystal photographs of 83~87 under sunlight (top) and room temperature 365nm UV light (bottom) and non-luminous (300 K, top) and phosphorescent (77 K, bottom) photographs of 87 nitrogen degassed cyclohexane solutions (10-4 mol/L) under 365nm UV lamp irradiation[5,170,173] |
图33 (a)98和99的化学结构;(b)98和99在氯仿和氩气脱氧氯仿溶液中被365nm紫外光照射下的RTP照片(2.0×10-5 mol/L);(c)98和99在日光下的薄膜图片(左)和在312nm紫外灯下(右)的薄膜RTP照片;(d)98在312nm紫外光照射前后的RTP余辉[191]Fig.33 (a) Chemical structures of 98 and 99. (b) RTP photographs of 98 and 99 exposed to 365nm UV light in trichloromethane and argon degassing trichloromethane solutions (2.0×10-5 mol/L). (c) Thin-film photographs of 98 and 99 in daylight (left) and thin-film RTP photographs in 312nm UV light (right). (d) RTP afterglow of 98 before and after 312nm UV irradiation[191] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
| [173] |
|
| [174] |
|
| [175] |
|
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
|
| [180] |
|
| [181] |
|
| [182] |
|
| [183] |
|
| [184] |
|
| [185] |
|
| [186] |
|
| [187] |
|
| [188] |
|
| [189] |
|
| [190] |
|
| [191] |
|
| [192] |
|
| [193] |
|
/
| 〈 |
|
〉 |