

Advances and Challenges of Low-Temperature Electrolyte for Sodium-Ion Batteries
Received date: 2023-03-21
Revised date: 2023-05-07
Online published: 2023-06-12
Supported by
National Natural Science Foundation of China(22075064)
China Postdoctoral Science Foundation(2022M710950)
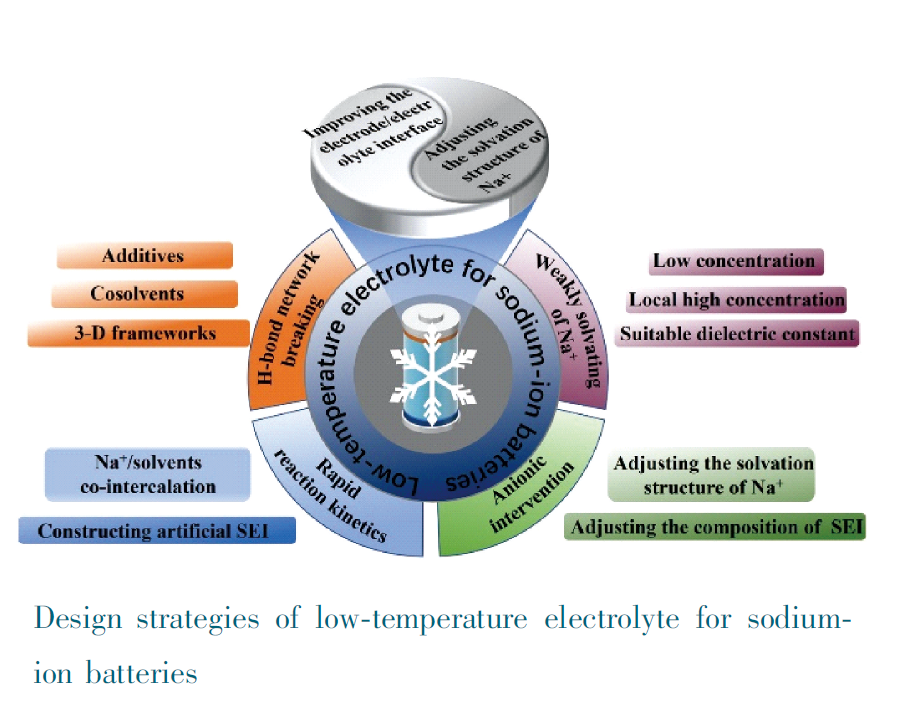
Sodium-ion batteries have attracted ever-increasing attention in the fields of low-speed electric vehicles, and large-scale energy storage systems due to the advantages of abundant resources, low cost, high safety, and environmental friendliness. As one of the important components of sodium-ion batteries, the electrolyte is responsible for ion transfer between the cathode and the anode, which has a significant impact on cycle life, high-rate, safety, and self-discharge performance of sodium-ion batteries. However, it is difficult for sodium-ion batteries to perform well at low temperatures due to the decrease in ionic conductivity, the poor compatibility between the electrolyte and the electrode, the increase of desolvating power, and the poor properties of the electrode/electrolyte interphase. In this paper, the new understanding of the Na+ solvation structure in the electrolyte and the electrode/electrolyte interphase in recent years are summarized. And the design strategies of low-temperature electrolyte based on H-bond network breakdown, weak solvation, rapid reaction kinetics, and anion intervention are systematically analyzed. Finally, it is pointed out that the key to improving the low-temperature performance of sodium-ion batteries from the perspective of electrolyte is to understand the relationship between the Na+ solvation structure, the electrode/electrolyte interface properties, and the low-temperature performance of electrolyte.
1 Introduction
2 Working principle of sodium-ion batteries and limitation of low-temperature performance of the electrolyte
3 Research status of low-temperature electrolyte for sodium-ion batteries
3.1 Design strategies of low-temperature electrolyte based on the H-bond network breaking method
3.2 Design strategies of low-temperature electrolyte based on weakly solvating
3.3 Design strategies of low-temperature electrolyte based on rapid reaction kinetics
3.4 Design strategies of low-temperature electrolyte based on anionic intervention
3.5 Others
4 Conclusion and outlook
Guangxiang Zhang , Chi Ma , Chuankai Fu , Ziwei Liu , Hua Huo , Yulin Ma . Advances and Challenges of Low-Temperature Electrolyte for Sodium-Ion Batteries[J]. Progress in Chemistry, 2023 , 35(10) : 1534 -1543 . DOI: 10.7536/PC230319
图3 基于破坏氢键网络方法的低温电解质设计策略. (a) χDMSO=0.3时溶剂结构模拟[30]; (b) NTP|H2O-DMSO|AC全电池在-50℃下的倍率性能[30], Copyright 2019, Wiley; (c) AC‖Na2CoFe(CN)6 全电池在-30℃下的电化学性能[33], Copyright 2022, WileyFig.3 Design strategies of low-temperature electrolyte based on H-bond network breaking method. (a) Solvent structure simulation with χDMSO=0.3[30]; (b) High-rate performance of NTP|H2O-DMSO|AC full-cell at -50℃[30], Copyright 2019, Wiley; (c) Electrochemical performance of AC‖Na2CoFe(CN)6 full-cell at -30℃[33], Copyright 2022, Wiley. |
表1 常见醚类溶剂[35⇓⇓~38]和羧酸酯类溶剂的物化性质[39⇓⇓~42]Table 1 Physicochemical properties of common ether solvents[35⇓⇓~38] and carboxylate solvents[39⇓⇓~42] |
| Solvent | Melting temperature Tm/℃ | Boiling temperature Tb/℃ | Viscosity η/ ( m Pa·s) (25℃) | Dielectric comstant (25℃) |
|---|---|---|---|---|
| Ethylene glycol dimethyl ether (DME) | -58 | 84 | 0.46 | 7.18 |
| Diethylene glycol dimethyl ether (DEGDME) | -64 | 162 | 1.06 | 7.4 |
| Tetraethyleneglycol dimethyl ether (TEGDME) | -46 | 111 | 3.39 | 7.53 |
| 1, 3-Dioxolane (DOL) | -95 | 74 | 0.59 | 6.79 |
| Tetrahydrofuran (THF) | -108 | 65 | 0.46 | 7.52 (22℃) |
| Methyl acetate (MA) | -84 | 57 | 0.36 | 6.68 |
| Ethyl acetate (EA) | -84 | 77 | 0.45 | 6.02 |
| Ethyl propionate (EP) | -74 | 99 | 0.5 | 5.76 (20℃) |
| Ethyl butyrate (EB) | -93.3 | 121.3 | — | — |
| [1] |
|
| [2] |
|
| [3] |
(尹成果, 马玉林, 程新群, 尹鸽平. 化学进展, 2013, 25(1): 54.).
|
| [4] |
(张玲玲, 马玉林, 杜春雨, 尹鸽平. 化学进展, 2014, 26 (4): 553.).
|
| [5] |
(李慧, 吴川, 吴锋, 白莹. 化学学报, 2014, 72(01): 21.).
|
| [6] |
(陆雅翔, 赵成龙, 容晓晖, 陈立泉, 胡勇胜. 物理学报, 2018, 67(12): 39.).
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
(殷秀平, 赵玉峰, 张久俊. 电化学, 2023, 1.).
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
/
| 〈 |
|
〉 |