

Application of Pyrite and Its Modified Composite in Water Pollution Treatment
Received date: 2023-02-16
Revised date: 2023-04-18
Online published: 2023-08-07
Supported by
National Natural Science Foundation of China(22206061)
Fundamental Research Funds for the Central Universities(JUSRP122022)
Due to its strong surface activity, precipitation adsorption, redox and relatively excellent photocatalytic properties, pyrite has been widely used to treat heavy metals, organic pollutants and various inorganic salts in the polluted water. However, some inherent defects of pyrite, such as small specific surface area, high susceptibility to agglomeration, etc., limit its practical applications. Appropriate modification of pyrite via morphological adjustment, elemental doping, and material loading can improve the dispersion performance of particle size, expose more functional groups and increase electron transport rate to further modulate the related properties and enhance the wastewater treatment capacity of pyrite, In this article, we firstly introduce the basic information, the application and the mechanism of pyrite in wastewater treatment, and then describe the typical modification methods of pyrite and their corresponding strengthening mechanisms for treating wastewater. This article will provide a systematic introduction and outlook for the development of pyrite-based composite materials in the field of environmental treatment.
1 Introduction
2 Adsorption of pyrite
2.1 Application and mechanism of pyrite adsorption capacity
2.2 Improvement of pyrite materials and enhancement of adsorption capacity
3 Oxidation of pyrite
3.1 Application and mechanism of pyrite oxidation ability
3.2 Improvement of pyrite materials and enhancement of oxidation capacity
4 Reduction of pyrite
4.1 Application and mechanism of pyrite reduction ability
4.2 Improvement of pyrite materials and enhancement of reduction capacity
5 Conclusion and outlook
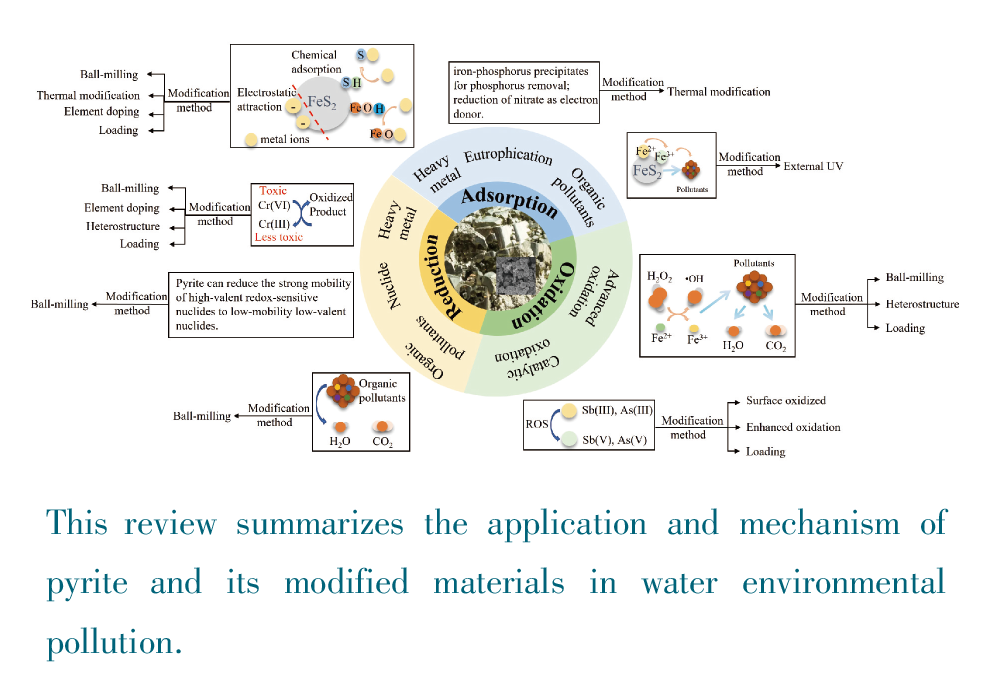
Key words: pyrite; modification; functional regulation; water pollution treatment
Yanxiao Chi , Yuxuan Yang , Kunlun Yang , Xianrong Meng , Wei Xu , Hengfeng Miao . Application of Pyrite and Its Modified Composite in Water Pollution Treatment[J]. Progress in Chemistry, 2023 , 35(10) : 1544 -1558 . DOI: 10.7536/PC230215
表1 黄铁矿改性材料吸附去除重(类)金属Table 1 Adsorption and removal of heavy metals by pyrite modified materials |
| Modification method | Modification material | Target metal | Removal performance | ref |
|---|---|---|---|---|
| Ball-milling | BM-ZVI/FeS2 | Sb(V) | 134 mg/g | 20 |
| BM-FeS2 | Pb(Ⅱ) | 34.10 mg/g | 42 | |
| BM-FeS2 | Cr(Ⅵ),Cd(Ⅱ),Pb(Ⅱ) | 4.75 mg/g, 2.87 mg/g, 4.91 mg/g | 46 | |
| Thermal modification | SV-FeS2 | Ni(Ⅱ) | 6.45 mg/g | 21 |
| FeS2/α-Fe2O3 | Sb(V) | 347.2 mg/g | 43 | |
| Element doping | Ni-FeS2 | Se(Ⅳ) | 15.79 mg/g | 44 |
| Loading | PY+AC-FA | Hg(Ⅱ) | 239.26 μg/g | 45 |
表2 黄铁矿改性材料催化氧化去除重(类)金属Table 2 Removal of heavy metals by oxidation of pyrite modified materials |
表3 改进型黄铁矿催化氧化降解有机物Table 3 Modified pyrite catalytic oxidation degradation of organic compounds |
| Modification method | Modification material | Target metal | Removal performance | ref |
|---|---|---|---|---|
| Ball-milling | Pyrite nanoparticles | Acid orange 7 | 16 mg/g | 77 |
| nano-pyrite | Sulfadiazine | 10 mg/g | 82 | |
| Heterostructure | FeS2/Fe2O3+TA | Carbamazepine | 1.3 mg/g | 78 |
| TiO2/FeS2 | Methylene blue | 6.1 mg/g | 83 | |
| Fe3O4@FeS2@C@MoS2 | Tetracycline | 12.5 mg/g | 84 | |
| ZnCo2O4/MnO2/FeS2 | Methyl orange | 3.84 mg/g | 85 | |
| FeS2/rGO | Methylene blue | 41.67 mg/g | 86 | |
| FeS2-Fe1-xS | Acid orange 7 | 15 mg/g | 87 | |
| CuO-FeS2 | Brilliant green | 2 mg/g | 88 | |
| Loading | FeS2/H2O2+AC, BC, CNTS | Ciprofloxacin | 89 mg/g, 71 mg/g, 68 mg/g | 80 |
表4 黄铁矿改性材料还原去除重金属Table 4 Removal of heavy metals by reduction with pyrite modified materials |
| Modification method | Modification material | Target metal | Removal performance | ref |
|---|---|---|---|---|
| Ball-milling | BM-FeS2@BC | Cr(Ⅵ) | 134 mg/g | 103 |
| Element doping | Ni-FeS2/FeS2 | Cr(Ⅵ) | 40 mg/g | 104 |
| Heterostructure | FeS2/Fe2O3 | Cr(Ⅵ) | 37.5 mg/g | 6 |
| α-FeOOH/FeS2 | Cr(Ⅵ) | 25 mg/g | 105 | |
| Loading | FeS2+Sepiolite | Cr(Ⅵ) | 14.27 mg/g | 102 |
| FeS2/Fe0 | Cr(Ⅵ) | 16.67 mg/g | 106 | |
| FeS2/biochar | Cr(Ⅵ) | 10 mg/g | 107 | |
| pyrite-marcasite-magnetite | Cr(Ⅵ) | 50 mg/g | 108 |
| [1] |
|
| [2] |
(罗宿星, 陈华仕, 牟青松, 伍远辉. 矿产综合利用, 2020, (05): 27.).
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
(栗占锋. 绍兴文理学院博士论文, 2017.).
|
| [8] |
(王遥. 吉林大学博士论文, 2022).
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
(石松, 吴乾元, 李新正, 黄满红. 环境科学, 2020, 41(09): 4124.).
|
| [15] |
|
| [16] |
|
| [17] |
(何叶. 南华大学博士论文, 2019.).
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
(胡国良. 华中师范大学博士论文, 2022.).
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
(王菊, 陈天虎, 李平, 谢晶晶, 马炳德, 曹光跃. 矿物学报, 2012, 32(02): 238.).
|
| [27] |
|
| [28] |
(张菁, 李睿华, 李杰, 刘波. 环境工程学报, 2013, 7(10): 3856.).
|
| [29] |
|
| [30] |
|
| [31] |
(王丹, 刘宏芳, 钱天伟. 环境污染与防治, 2014, 36(03): 30.).
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
(方艳芬, 李新玉, 周薇, 王小维, 蔡宽, 贾漫珂, 黄应平. 环境化学, 2014, 33(11): 1941.).
|
| [38] |
(蔡宽, 熊世威, 张欣欣, 李瑞萍, 黄应平. 岩石矿物学杂志, 2014, 33(02): 370.).
|
| [39] |
(胡俊松, 李睿华, 孙茜茜, 刘卓, 张小梅. 环境工程学报, 2015, 9(11): 5463.).
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
(黄树杰. 广东工业大学博士论文, 2017.).
|
| [45] |
|
| [46] |
(崔晋艳. 太原科技大学博士论文, 2016.).
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
(郭迪满. 华中农业大学博士论文, 2021.).
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
(王洪红, 雷文, 李孝建, 黄仲, 贾全利, 张海军. 化学进展, 2021, 32(12): 1990.).
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
(刘宏芳, 钱天伟, 张敏刚. 光谱学与光谱分析, 2015, 35(02): 543.).
|
| [96] |
(李平. 合肥工业大学博士论文, 2016.).
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
(黄海军, 陈金毅, 王小凤. 现代矿业, 2021, 37(04): 176.).
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
/
| 〈 |
|
〉 |