

Design and Synthesis of Degradable Polyolefins
Received date: 2023-03-01
Revised date: 2023-04-13
Online published: 2023-08-06
Supported by
The National Natural Science Foundations of China(51973224)
The National Natural Science Foundations of China(52173013)
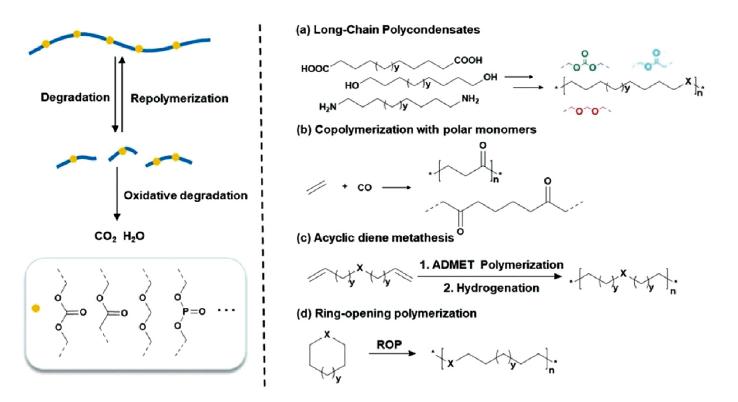
Polyolefin is thermoplastic universal plastic widely used in daily life. However, the overuse of polyolefin plastic and lack of degradability has led to a large amount of plastic waste, as well as growing land and marine pollution problems. The overwhelming majority of post-consumer polyolefin plastic is not recycled. Obstacles to the recycling of waste plastic include high energy consumption, low utilization rate of recycled products, low added value, and other wastes generated in the recycling process. Polyolefins degrade very slowly in the environment, and the addition of co-degraders can also cause environmental pollution. A feasible alternative is to redesign and synthesize degradable polyolefins, which can solve waste plastic problem from the source. The synthesis of degradable polyolefins has been extensively studied over the past half century. This paper summarizes the degradation mechanism of polyolefins, including oxidative degradation and co-degradation technology. Meanwhile we review four approaches to synthesizing degradable polyolefins, which cover condensation of long-chain bifunctional monomers, copolymerization with polar monomers, acyclic diene metathesis, and ring-opening polymerization. Among them, olefin metathesis polymerization has significantly expanded the types of degradable polyolefins due to the superior tolerance of the catalysts to functional groups, such as polyester, polyacetal, polycarbonate, polyphosphoester. We discuss the forward-looking synthetic approaches offered by current research and the challenges that these degradable materials face in truly replacing polyolefin materials. Finally, we propose our perspective on the opportunities and challenges in this field.
1 Introduction
2 Degradation mechanism of polyolefin
2.1 Oxidative degradation
2.2 Co-degradation technology
3 Synthesis of degradable polyolefins
3.1 Polycondensation of long chain difunctional monomers
3.2 Copolymerization with polar monomers
3.3 Acyclic diene metathesis
3.4 Ring-opening polymerization
4 Conclusion and outlook
Huiping Yu , Yawei Qin , Jinyong Dong . Design and Synthesis of Degradable Polyolefins[J]. Progress in Chemistry, 2023 , 35(9) : 1294 -1303 . DOI: 10.7536/PC230229
图3 (a)具有不同亚甲基序列长度的聚酯材料与LLDPE、聚己二酸/对苯二甲酸丁二醇酯(PBAT)在力学性能、结晶性、O2阻隔性的比较;(b)固定x时聚酯 过冷温度ΔT和熔化焓ΔHm对二酸链长的依赖性[22]Fig.3 (a) Comparison of mechanical properties, crystallinity and O2 barrier between polyester materials with different methylene sequence lengths and LLDPE, polyadipate/butylene terephthalate (PBAT); (b) diacid chain length dependence of supercooling temperature ΔT and melting enthalpies ΔHm of polyester with fixed x.[22] Copyright 2021, ACS. |
表1 通过ADMET反应合成的可降解聚合物总结Table 1 Summary of representative degradable polymers via ADMET |
图4 (a)基于衣康酸和10-十一烯醇的高分子量聚酯的合成、功能化和可控降解[48];(b)通过ADMET反应制备木糖基聚醚/聚酯[50]Fig.4 (a)Synthesis, functionalization and controlled degradation of high molecular weight polyesters based on iticonic acid and 10-undecenol[48]. Copyright 2014, ACS. (b) Xylose-based polyethers and polyesters via ADMET polymerization toward polyethylene-like materials[50]. Copyright 2021, ACS |
图5 通过各种链生长机制的开环聚合合成可降解聚合物及代表性单体。(a) 环烯酮缩醛和硫代内酯的自由基ROP;(b) 阴离子/阳离子/金属/有机催化ROP,以阴离子催化ROP机理为代表;(c) 环烯烃单体的ROMPFig.5 Synthetic degradable polymers through ring-opening polymerization via various chain-growth mechanisms. (a) Radical ROP of cyclic ketene acetals and thionolactone; (b) Anionic/cationic/metal/organo-catalyzed ROP, anion catalyzed ROP mechanism is represented; (c) ROMP of cyclic olefin monomers |
| [1] |
|
| [2] |
|
| [3] |
(刘朝艳. 塑料工业, 2022, 50(04): 1.).
|
| [4] |
|
| [5] |
|
| [6] |
(刘雪辉, 徐世美, 张帆, 汪秀丽, 王玉忠. 高分子学报, 2022, 9: 1005.).
|
| [7] |
|
| [8] |
|
| [9] |
(骆希, 詹佳慧, 张士成. 能源环境保护, 2023, 37(1): 194.).
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
(宫涛. 精细与专用化学品, 2014, 22(6): 11.).
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
/
| 〈 |
|
〉 |