

Strigolactone and Its Novel Derivatives
Received date: 2023-01-02
Revised date: 2023-05-24
Online published: 2023-08-06
Supported by
The National Key R&D Program Projects of China(2020YFA0907903)
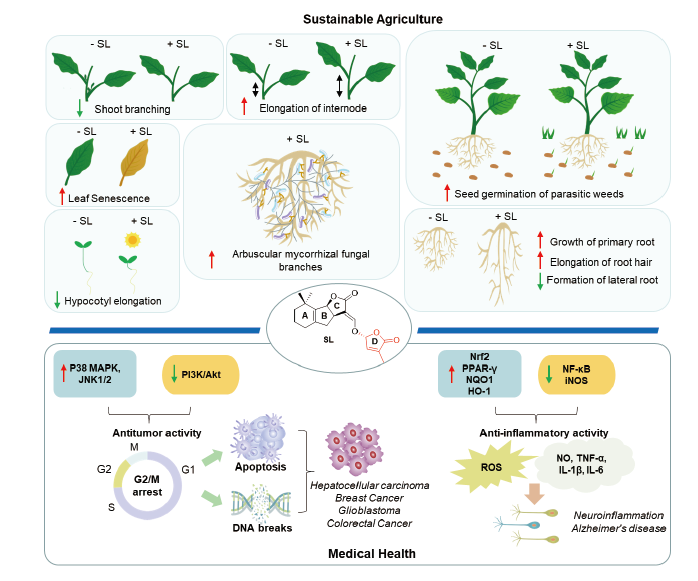
Strigolactones (SLs) are the most concerned endogenous sesquiterpenoid phytohormones.Recent studies have shown that strigolactones play crucial roles in inhibition of plant hypocotyl elongation and crop tillering, regulating root growth and development, stimulation of parasitic weed seed germination, coordinating the symbiotic interaction between parasitic plants and fungi, as well as regulation of plant response to biotic or abiotic stresses. Therefore, it is considered to be a new type of phytohormone with great development value and application potential in the field of agricultural science and plant protection. In addition, SL derivatives have also attracted much attention in the field of innovative drug research as the studies have found that: (1) SLs exhibit inhibitory activities against several tumor cell lines such as liver cancer, breast cancer, prostate cancer, glioblastoma, and colorectal cancers; (2) they possess anti-inflammation and glucose metabolism inhibitory activity. This paper aims to review the latest research progress of strigolactone and its structural derivatives with brief analysis on their biological activity, mechanism of action and structure-activity relationship. We hope this review provide guidance and directions on molecular design, development and utilization of SL natural products.
1 Introduction
2 Structural features and classification of strigolactones
3 The biosynthetic pathway and signal transduction mechanism of strigolactones
4 Structural characteristics and classification of natural strigolactones
5 Structural characteristics and classification of synthetic strigolactones
5.1 Canonical derivatives of strigolactone
5.2 Non-canonical derivatives of strigolactone
6 Conclusion and outlook
Zhaoyong Kang , Xiaoqi Dong , Shengnan Liu , Qingzhi Gao . Strigolactone and Its Novel Derivatives[J]. Progress in Chemistry, 2023 , 35(9) : 1341 -1356 . DOI: 10.7536/PC221234
表1 天然独脚金内酯的分类和生物活性Table 1 Classification and biological activity of natural strigolactones |
| Classification | SLs name | Plant source | Biological activity | Action object & biotarget | ref |
|---|---|---|---|---|---|
| Strigol-tpye SLs | |||||
| Canonical SLs | Strigol (1) | Cotton, Menispermum dauricum | germination stimulant hyphal branching inducers inhibit shoot branching anti-inflammation, anti-cancer | Striga, Orobanche arbuscular mycorrhizal fungi Rice Nrf2, NF-κB | 16~18 |
| 5-Deoxystrigol (5DS, 2) | Cotton, Chinese milk vetch, Sorghum | hyphal branching inducers inhibit shoot branching liver injury protection | arbuscular mycorrhizal fungi Rice Nrf2 | 4,19~21 | |
| Strigone (3) | Houttuynia cordata | germination stimulant anti-hepatic fibrosis | O. minor, P. ramosa, S. hermonthica TGF | 22 | |
| Sorgolactone (4) | Sorghum | germination stimulant | Striga, Orobanche | 23 | |
| Sorgomol (5) | Sorghum | germination stimulant | Striga, Orobanche | 24,25 | |
| Orobanchol-type SLs | |||||
| Orobanchol (ORO, 6) | Red clover, Rice, Tobacco | germination stimulant | O. minor, P. ramosa | 26,27 | |
| 4-Deoxyorobanchol (4DO, 7) | Rice | germination stimulant | O. minor | 28,29 | |
| Orobanchol acetate (8) | Cowpea, Soybean, Red clover | germination stimulant | O. minor, O. ramosa | 30,31 | |
| 7-Oxoorobanchyl acetate (9) | Flax | germination stimulant | O. minor | 32 | |
| 7-Oxoorobanchol (10) | Flax | germination stimulant | O. minor | 32 | |
| Solanacol (11) | Tobacco | germination stimulant | O. minor | 26,33,34 | |
| Fabacyl acetate (12) | Pea, Faba bean, Alfalfa | germination stimulant | O. minor | 35 | |
| Medicaol (13) | Medicago truncatula | hyphal branching inducers | arbuscular mycorrhizal fungi | 36 | |
| Non-canonical SLs | Carlactonate (CL, 14) | Sunflower | germination stimulant inhibit shoot branching | S. hermonthica Rice | 37 |
| Carlactonoic acid (CLA, 15) | Rice, Arabidopsis thaliana, Selaginella | inhibit shoot branching | Arabidopsis thaliana | 38,39 | |
| Methyl carlactonate (MeCLA, 16) | Sunflower | hyphal branching inducers | Gigaspora margarita | 40,41 | |
| Methyl heliolactonate (17) | Sunflower | germination stimulant | S. hermonthica | 42 | |
| Avenaol (18) | Black oat | germination stimulant | P. ramose, S.hermonthica, O. minor | 43 | |
| Methyl zealactonate (19) | Maize | germination stimulant | O. minor, P. ramosa, S. hermonthica | 44,45 | |
| Lotuslactone (20) | Lotus japonicus | hyphal branching inducers germination stimulant | arbuscular mycorrhizal fungi O. minor, P. ramosa, S. hermonthica | 46 | |
| Cannalactone (21) | Cannabis sativa | germination stimulant | P. ramosa | 47 | |
| Bryosymbiol (22) | Marchantia paleacea | hyphal branching inducers germination stimulant | arbuscular mycorrhizal fungi O. minor, P. ramosa, S. hermonthica | 48 | |
图11 化合物80的结构与分子机制。(A)化合物80的分子结构;(B)化合物80与拟南芥AtD14蛋白的分子结合模式 (PDB: 4IH4);(C)化合物80与拟南芥AtD14蛋白的复合结构;(D)化合物80与ShHTL7蛋白的复合结构 (PDB: 5Z7Y)Fig.11 Structure and molecular mechanism of compound 80. (A) Molecular structure of compound 80; (B) Molecular binding mode of compound 80 to Arabidopsis AtD14 protein (PDB: 4IH4); (C) Complex structure of compound 80 with Arabidopsis AtD14 protein; (D) Complex structure of compound 80 with ShHTL7 protein (PDB: 5Z7Y) |
图13 独脚金内酯类似物其他类的代表性分子结构96~112Fig.13 Structures of other types of strigolactone analogs 96~112 |
图14 化合物96的分子机制。(A)化合物96与ShHTL7的分子结合模式 (PDB: 5Z7Y);(B)化合物96与拟南芥AtD14的分子结合模式 (PDB: 4IH4);(C)化合物96与ShHTL7的复合结构;(D)化合物96与AtD14的复合结构Fig.14 Molecular mechanisms of compound 96. (A) Molecular binding mode of compound 96 to ShHTL7 (PDB: 5Z7Y); (B) Molecular binding mode of compound 96 to Arabidopsis AtD14 (PDB: 4IH4); (C) Complex structure of compound 96 with ShHTL7 protein; (D) Complex structure of compound 96 with AtD14 protein |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
/
| 〈 |
|
〉 |