

Photocatalytic Removal of Antibiotics from Water
Received date: 2023-05-25
Revised date: 2023-08-19
Online published: 2023-09-10
Supported by
Major Scientific and Technological Innovation Project of Shandong Province(2021CXGC011206)
With the widespread use of antibiotics, the problem of water pollution caused by antibiotics is becoming increasingly serious. Currently, technologies for removing antibiotic pollutants from water include physical adsorption, flocculation, and chemical oxidation. However, these processes often leave a large amount of chemical reagents and difficult-to-dispose sediment in water, making post-treatment more difficult. Photocatalytic technology uses photocatalytic materials to decompose antibiotics under light, ultimately forming non-toxic CO2 and H2O. Photocatalytic degradation of antibiotics has the advantages of low cost, high efficiency and free secondary pollution. In this paper, the research progress of several commonly used photocatalytic materials for degrading antibiotics is reviewed, and their future researches and applications are also prospected.
1 Introduction
2 the impact of antibiotics in wastewater on The environment and human health
3 Principles of photocatalytic degradation of antibiotics
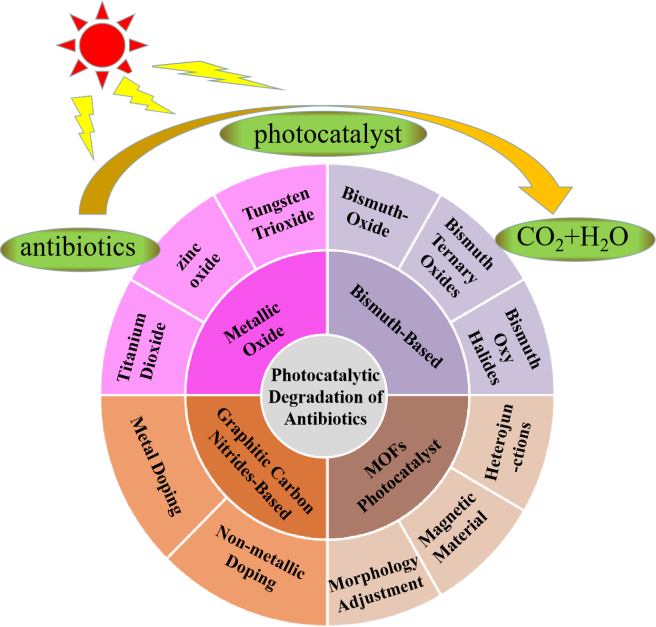
4 Commonly used photocatalytic materials for antibiotic degradation
4.1 Metal oxide based photocatalyst
4.2 Bi-based photocatalysts
4.3 Photocatalysts based on metal organic frameworks(MOFs)
4.4 Graphite like g-C3N4photocatalyst
5 Photocatalytic degradation of antibiotics
5.1 Photocatalytic degradation of TC
5.2 Photocatalytic degradation of CIP
6 Conclusion and outlook
Key words: photocatalyst; antibiotic; degradation; electronic energy band structure
Jiangbo Yu , Jing Yu , Jie Liu , Zhanchao Wu , Shaoping Kuang . Photocatalytic Removal of Antibiotics from Water[J]. Progress in Chemistry, 2024 , 36(1) : 95 -105 . DOI: 10.7536/PC230525
表1 不同催化剂降解抗生素及其降解效果Table 1 Degradation of antibiotics with different catalysts and their degradation effects |
| Antibiotic | Photocatalyst | Result | Degradation mechanism | ref |
|---|---|---|---|---|
| TC | Heterogeneous TiO2/g-C3N4 | 100 mg TiO2/g-C3N4 can decompose 20 mg tetracycline (2.2 mg/min) within 9 min | Co action of ·O2− and h+ | 71 |
| TC | Heterogeneous AgI/BiVO4 | The degradation rate of TC within 70 min is 94.91% | Co action of ·OH,·O2− and h+ | 70 |
| TC | 3D polymerized carbon nitride foam | TC degradation rate in 70 min seawater 78.9% | Co action of ·O2− and h+ | 5 |
| TC | ARCNS-3 | TC degradation rate within 5 h is 100% | Inhibition of photogenerated electrons and free radical intermediates by aromatic rings | 7 |
| TC | S-scheme In2Se3/Ag3PO4 | 1 h TC degradation rate 93.1% | Co action of ·OH,·O2− and h+ | 12 |
| TC | (Bi)BiOBr/rGO | 20 min degradation rate 98% | Co action of ·O2− and h+ | 43 |
| CIP | CuO:Zn | The degradation rate of CIP within 240 min is 94.6% | Synergistic effect of ·OH and h+ | 76 |
| CIP | Flaky peeling g-C3N4 | The degradation rate of CIP within 60 min is 78% | Co action of ·O2− and h+ | 77 |
| CIP | CZSNO20 | The degradation rate of CIP in 60 minutes is nearly 83% | Co action of e−, h+,·OH and ·O2− | 8 |
| CIP | Z-scheme Ag3PO4 @MoS2 | The degradation rate of CIP within 2 h reached 91.7% | Co action of ·OH,·O2− and h+ | 6 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
/
| 〈 |
|
〉 |