

Research and Application of Materials and Micro/Nano Structures for Light Manipulation
Received date: 2022-12-28
Revised date: 2023-04-15
Online published: 2023-07-18
Supported by
National Key Research and Development Program of China(No.2019Q(Y)Y)0503)
National Natural Science Foundation of China(91963104)
Technical Institute of Physics and Chemistry and Joint R&D Laboratory for Functional Agriculture Films.
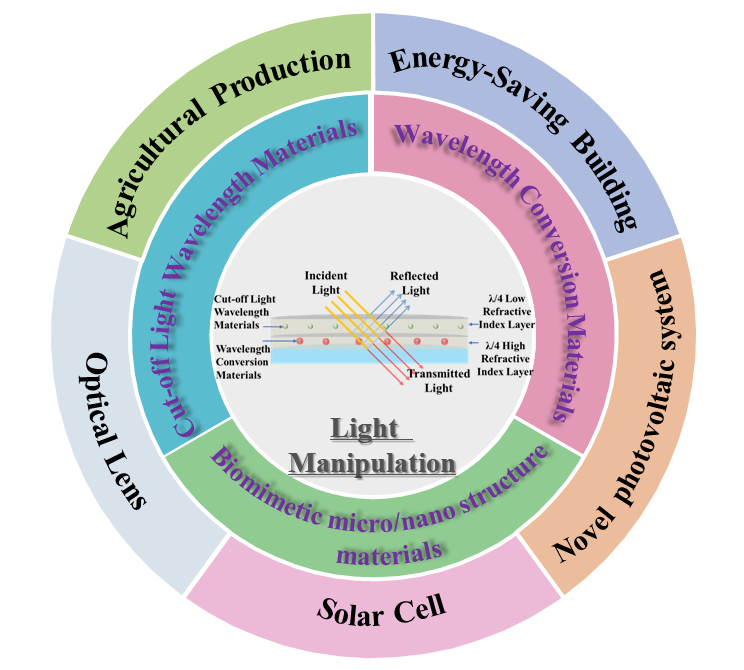
Solar energy, as one of the cleanest energy sources, is a precious resource endowed by nature to humanity. The solar spectrum and radiation intensity have a direct impact on human production and life, and how to utilize sunlight more efficiently has always been a goal pursued by scientists. This review systematically introduces the materials that can be used for light regulation, as well as their synthesis methods, and optical properties, including static light manipulation materials (such as UV shielding agents, visible light regulation materials, and infrared light regulation materials), stimulus responsive intelligent light manipulation materials (photoluminescence materials, intelligent color changing materials, etc.), and biomimetic micro/nanostructure materials. And further summarized the effects of light manipulation (including light wavelength, light intensity, and light propagation direction) that can be achieved using different types of light manipulation materials (micro nano structures). Finally, the current application status and development prospects of light manipulation materials and technologies in energy-saving buildings (including smart windows), agricultural films, solar photovoltaic power generation, and other fields were comprehensively summarized.
1 Introduction
2 Classification and optical properties of light manipulation materials
2.1 Static light manipulation materials
2.2 Stimulation-responsive intelligent light manipulation materials
2.3 Biomimetic micro/nano structural materials
3 Application of light manipulation materials and technology
3.1 Energy-saving building
3.2 Agricultural film
3.3 Photovoltaic power generation
4 Conclusion and outlook
Sainan Zhang , Cuixia Wu , Junhui He , Mingxian Wang , Shuangzhi Qin . Research and Application of Materials and Micro/Nano Structures for Light Manipulation[J]. Progress in Chemistry, 2023 , 35(8) : 1136 -1153 . DOI: 10.7536/PC221223
图3 (a) 碳点与半导体量子点的结构对比[64];(b) 荧光纳米点的分类[66];(c) 碳纤维通过化学氧化切割合成碳点(自上而下)的示意图[69];(d) 通过醛醇缩合反应自下而上法合成CD的示意图[74]Fig.3 (a) Comparison of structure of carbon dots and semiconductor quantum dots[64], Copyright 2021, American Chemical Society; (b) classification of fluorescent nanodots[66], Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim;(c) schematic diagram of carbon fiber cutting through chemical oxidation to synthesize CD (top-down)[69], Copyright 2012, American Chemical Society; (d) schematic diagram of CD synthesis by aldol condensation reaction from bottom to top[74], Copyright 2021, American Chemical Society |
图4 (a) 成熟蛾的照片及不同放大倍数下蛾眼的SEM图[106] ;(b) 仿生蛾眼纳米结构涂层的示意图(内),涂层的反射率及对应的折射率分布曲线[110];(c) 蓝宝石桡足类(Sapphirina metallina)的超微结构SEM图(紧密排列的六角形晶体及鸟嘌呤晶体和细胞质的交替层)[116] ;(d) 仿变色龙式热致变色智能窗的制备及机理示意图[117]Fig.4 (a) Photograph of the adult Philosamia cynthia ricini and SEM images of its moth-eye structure at different magnifications[106], Copyright 2016, American Chemical Society; (b) schematic diagram of bionic moth eye nanostructure coating (inset), reflectance of the coating and the corresponding refractive index distribution curve[110], Copyright 2017, American Chemical Society; (c) SEM images of ultrastructure of Sapphirina metallina (the tightly packed hexagonal crystals and the alternating layers of guanine crystals and cytoplasm beneath the procuticle)[116], Copyright 2015, American Chemical Society; (d) Schematic illustration of the preparation and mechanism of thermochromic smart windows[117], Copyright 2021, American Chemical Society |
图5 (a) 理想窗户的示意图,分别适用于夏季(ⅰ)和冬季(ⅱ)[123];(b) CsxWO3/PAM-PNIPAM智能窗的设计思路(ⅰ),a) CsxWO3/PAM-PNIPAM智能窗的制备示意图,b) PNIPAM凝胶的流体动力学直径随温度的变化,c) PNIPAM微凝胶在水分散液中的流体动力学直径分布曲线(内插图为PNIPAM凝胶的SEM图)(ⅱ)[130]Fig.5 (a) Schematic diagram of perfect windows for summer (ⅰ) and winter (ⅱ)[123], Copyright 2014, American Chemical Society; (b) designing scheme of the CsxWO3/PAM-PNIPAM smart window (ⅰ), a) scheme of the fabrication of CsxWO3/PAM-PNIPAM window, b) hydrodynamic diameter of PNIPAM microgels with temperature, c) hydrodynamic diameter distribution curve of the PNIPAM microgels in aqueous dispersion (Inset: the SEM image of the PNIPAM microgels) (ⅱ)[130], Copyright 2018, American Chemical Society |
图6 (a) (ⅰ) 三苯基丙烯腈发光剂的合成方案,(ⅱ) 六种发光剂分别在溶剂、固体和PVC薄膜中的发光性能和荧光量子产率[141];(b) CaBr2, CaF2掺杂的CaS:Eu2+的稳定性和应用示意图[144];(c) 核壳结构CaS:Eu2+,Pb2+@CaZnOS:Pb2+的合成及光谱转换性能示意图[145]Fig.6 (a) (ⅰ) Synthetic schemes of the triphenyl acrylonitrile luminous agent, (ⅱ) luminescent properties and fluorescence quantum yields of the six luminescent agents in solvent, solid and PVC films respectively[141], Copyright 2018, American Chemical Society; (b) schematic diagram of the stability and application of CaS:Eu2+,CaBr2,CaF2 composite phosphor[144], Copyright 2021, American Chemical Society; (c) schematic diagram of synthesis and spectral conversion performance of the core-shell structured CaS:Eu2+,Pb2+@CaZnOS:Pb2+[145], Copyright 2022, American Chemical Society |
图7 (a) 具有微圆顶结构的薄膜的示意图;(b) 具有微圆顶结构的转光剂掺杂薄膜的横截面示意图;(c) 具有微圆顶结构及不具有微结构转光膜与普通薄膜的前向光谱辐照度[153]Fig.7 (a) Schematic diagram of the film with micro-dome structure; (b) cross-section schematic of the phototransfer-doped film with micro-dome structure; (c) forward spectral irradiance of light transfer films with and without micro-dome structure and ordinary films[153], Copyright 2022, Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement |
图8 (a) AM1.5G太阳能分布(黑线),理想的全色吸收光谱(绿线);(b) 染料敏化太阳能电池的运行机制[160]; (c) 钙钛矿太阳能电池中下转换材料层的设计[166];(d) 基于PbSe油墨的太阳能电池的J-V曲线(内插图:(ⅰ) 一步法合成PbSe量子点油墨的过程),(ⅱ) 基于PbSe油墨的太阳能电池的结构示意图[174];(e) 二维IOP-1000光子薄膜的SEM图(内插:该薄膜的数码照片),基于IOP-500和IOP-1000 MAPbI3薄膜的太阳能电池的J-V特征曲线[181]Fig.8 (a) AM1.5G solar energy distribution (black curve), ideal panchromatic absorption spectrum (green curve); (b) Operational mechanism of dye-sensitized solar cells[160], Copyright 2019, American Chemical Society; (c) Schematic diagram of down conversion layer in perovskite solar cells[166], Copyright 2022 The Authors. Solar RRL published by Wiley-VCH GmbH; (d) J-V curve of optimized PbSe QD device (inset: (ⅰ) “one-step” direct synthesis of PbSe QD inks), (ⅱ) scheme of the device architecture with PbSe QD inks[174], Copyright 2020, American Chemical Society; (e) the SEM images of 2D IOP-1000 photonic film (inset: the digital photo of the IOP-1000 film), J-V characteristics of the SCs based on the IOP-500 and IOP-1000 MAPbI3 films[181], Copyright 2016, American Chemical Society |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
(陈华湘. 吉林农业, 1995, 5: 20.).
|
| [139] |
(利双. 农村实用技术与信息, 1994, 4: 13.).
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
| [173] |
|
| [174] |
|
| [175] |
|
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
|
| [180] |
|
| [181] |
|
/
| 〈 |
|
〉 |