

Peracetic Acid-Based Advanced Oxidation Processes and Its Applications in Water Disinfection
Received date: 2022-12-28
Revised date: 2023-03-27
Online published: 2023-05-10
Supported by
National Natural Science Foundation of China(2019YFC0408801)
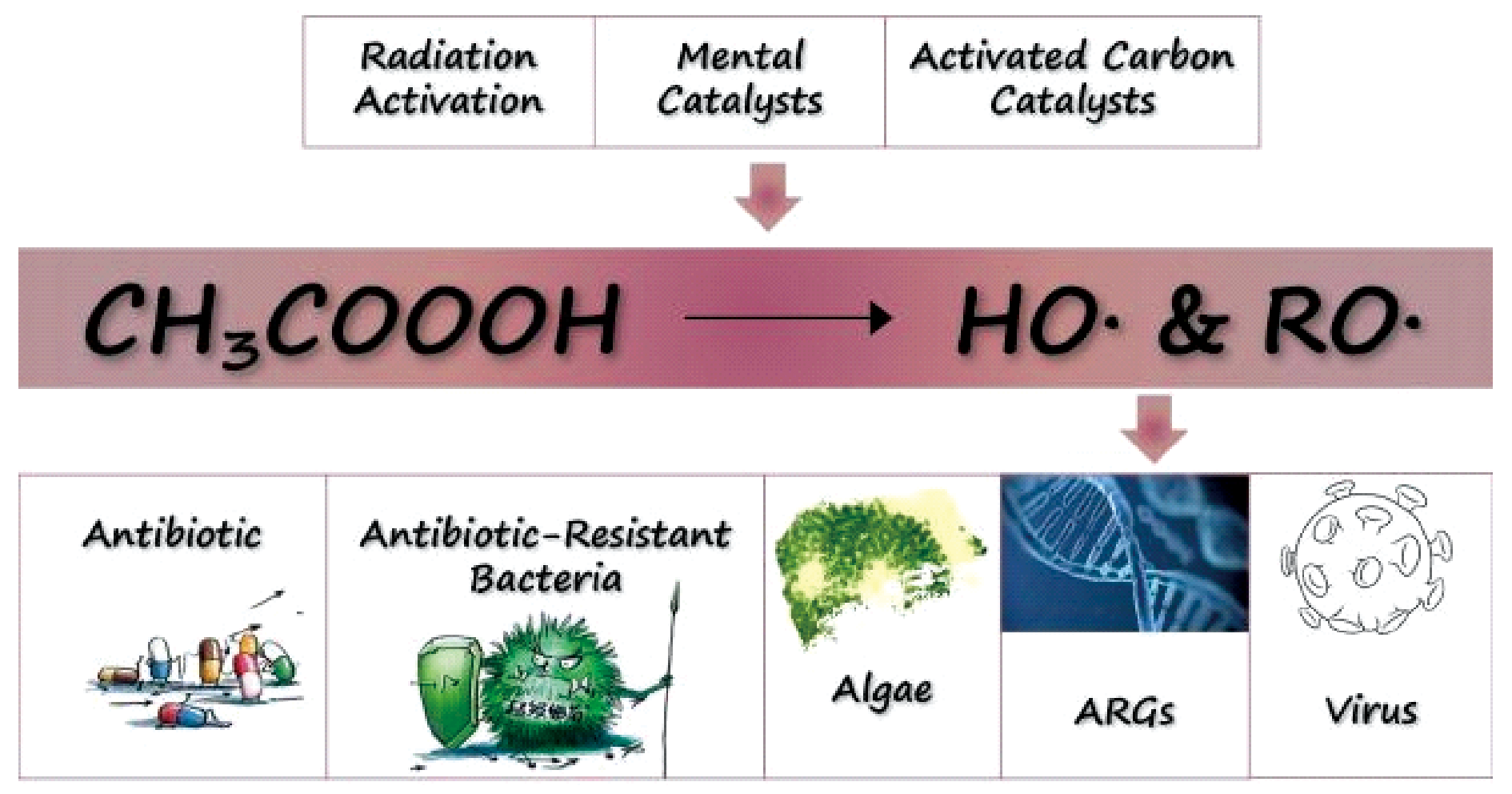
Recent research has revealed that PAA-based advanced oxidation processes (AOP) can simultaneously destroy developing micropollutants in water while having a greater disinfection efficacy than PAA alone. This paper summarizes the activation mechanism of PAA-based AOP and its use in water disinfection. According to recent study, UV/PAA has a good treatment effect in the cutting-edge problems of water disinfection, such as the removal of algae and algal toxins, the inactivation of fungus and antibiotic-resistant bacteria, etc. It is awaiting more investigation. There are few AOPs in the realm of water disinfection that activate PAA in other ways, but they have significant research promise. Identification of potential disinfection by-products found in AOP of PAA may also become a focus of future research.
1 Introduction
2 Peracetic acid-based advanced oxidation processes and activation mechanism
2.1 Radiation activation
2.2 Metal catalysts activation
2.3 Activated carbon catalysts activation
3 Recent advances of peracetic acid-based advanced oxidation processes in water disinfection
3.1 Recent advances of bacterial inactivation
3.2 Recent advances of fungus and algae inactivation
3.3 Recent advances of virus inactivation
3.4 Recent advances of DBPs
4 Conclusion and outlook
Yining Li , Minghao Sui . Peracetic Acid-Based Advanced Oxidation Processes and Its Applications in Water Disinfection[J]. Progress in Chemistry, 2023 , 35(8) : 1258 -1265 . DOI: 10.7536/PC221214
表1 基于PAA的AOP降解有机污染物的研究概述Table 1 Overview of studies employing PAA-based AOPs for removing organic compounds |
| Precesses | Target Contamiants | pH | kobs | Priaary Radicals | ref |
|---|---|---|---|---|---|
| UV/PAA | BZF | 7.1 | (2.40± 0.06)×10-2 min-1 | HO· | 46 |
| UV/PAA | DCF | 7.1 | 1.38± 0.03 min-1HO· | 46 | |
| UV/PAA | CA | 7.1 | (2.30±0.03)×10-1 min-1 | HO· | 46 |
| UV/PAA | IBP | 7.1 | (8.83±0.15)×10-2 min-1 | HO· | 46 |
| UV/PAA | NAP | 7.1 | (3.07±0.07)×10-1 min-1 | HO· | 46 |
| UV/PAA | CBZ | 7.1 | (8.25±0.31)×10-2 min-1 | HO· | 46 |
| Fe2+/PAA | MB | 3.0~8.2 | 0.17~1.75 s-1 | HO· | 27 |
| Fe2+/PAA | NPX | 3.0~8.2 | 0.45~2.33 s-1 | HO· | 27 |
| Fe2+/PAA | BPA | 3.0~8.2 | 0.21~0.75 s-1 | HO· | 27 |
| nCuO/PAA | CBZ | 7 | 0.07 min-1 | CH3C(O)OO· | 33 |
| ZVC/PAA | DCF | 2.0~11.0 | 0.1034~0.0017 min-1 | HO· | 35 |
| Cu2+-HCO3- (CO32-)/PAA | DCF | 9.3 | 0.0835 min-1 | RO· | 36 |
| nCo3O4/PAA | CBZ | 7 | 0.03 min-1 | RO· | 42 |
| Co2+/PAA | SMX | 7 | 0.1322 min-1 | CHSC(O)OO· | 37 |
| Co2+/PAA | BPA | 7 | (2.98±0.01)×10-4 (3.34±0.24)×10-2s-1 | CH3C(O) OO· | 38 |
| Co2+/PAA | NAP | 7 | (4.05±0.01)×10-4 (8.12±0.38)×10-2s-1 | CH3C(O) OO· | 38 |
| Co2+/PAA | SMX | 7 | (1.60±0.00)×10-4 (1.95±0.08)×10-2s-1 | CH3C(O) OO· | 38 |
| Co2+/PAA | CBZ | 7 | (2.32±0.03)×10-4 (3.23±0.07)×10-3s-1 | CH3C(O) OO· | 38 |
| Mn2+/PAA | Orange Ⅱ | 7 | 6.05×10-2s-1 | MnⅣ=O | 41 |
| MoS2/PAA | SMX | 7 | 0.128 min-1 | HO· | 26 |
| US/MnO2/PAA | Phenol | 7 | 0.89 min-1 | - | 24 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
(王传虎, 方荣生. 中国消毒学杂志, 2006, (2) 100.).
|
| [7] |
|
| [8] |
( 田秋霖. 湖北医学院学报, 1991, (1): 59.).
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
/
| 〈 |
|
〉 |