

Condensed Matter Chemistry in Catalysis by Zeolites
Received date: 2022-10-17
Revised date: 2022-12-31
Online published: 2023-02-20
Supported by
The National Natural Science Foundation of China(U21B20101)
The National Natural Science Foundation of China(22288101)
This work is devoted to condensed matter chemistry in gas-phase catalytic reactions over zeolite catalysts, which mainly involve in the processes of (i) adsorption of gaseous reactants into zeolite micropores, (ii) conversion of the reactants on catalytic sites in zeolites, and (iii) desorption of products in zeolites. In the above processes, both fast adsorption in zeolite micropores and rapid desorption from zeolites can significantly improve the reaction rate. To realize these purposes, it has been developed new strategies for rational synthesis of zeolites including preparation of zeolite nanocrystals, introduction of mesopores into zeolite crystals, preparation of zeolite nanosheets, and adjusting wettability of zeolite crystals, which have been simply concluded. Furthermore, the catalytically active sites including single atoms and metal nanoparticles can be introduced into zeolite frameworks or zeolite crystals, which can combine both the advantages of high stability and excellent shape selectivity for zeolites and the advantages of high activity and anti-deactivation for metal species together, offering a good opportunity to design and preparation of new highly efficient zeolite-based catalysts in the future. Finally, it is suggested perspectives such as rational synthesis of zeolite catalysts by theoretical simulations from the energy comparison, preparation of highly efficient catalysts by incorporating catalytically active sites in zeolite framework from the requirements of catalytic reactions, and green synthesis of zeolites for reduction of harmful gases, polluted water, and solid wastes in industrial processes.
1 Introduction
2 Adsorption of gaseous reactants in zeolite micropores
2.1 Preparation of zeolite nanocrystals
2.2 Introduction of mesoporosity in zeolite crystals
2.3 Preparation of zeolite nanosheets
3 Conversion of reactants on catalytic sites in zeolites
3.1 Acidic sites in zeolite frameworks
3.2 Heteroatoms in zeolite frameworks
3.3 Multisites in zeolite crystals
4 Desorption of products from zeolite catalysts
4.1 Preparation of zeolite nanocrystals and nanosheets and introduction of mesopores into zeolite crystals
4.2 Adjusting wettability of zeolite catalysts
4.3 Selective adsorption of reaction products by zeolite additives
5 Conclusion and perspectives
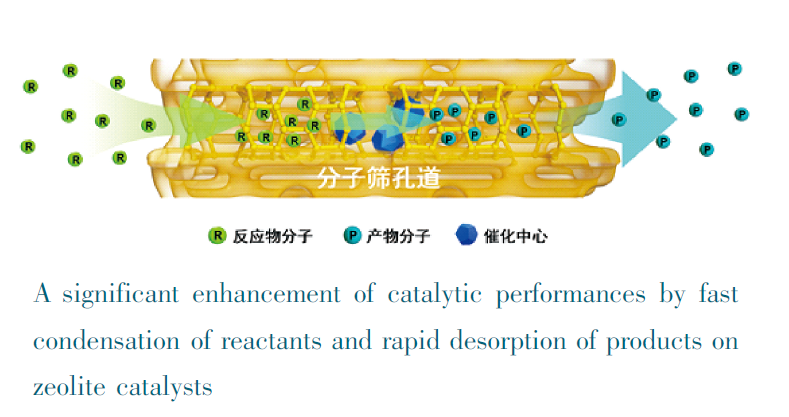
Key words: condensed matter; gas-solid phase catalysis; zeolite; catalytic materials
Fengshou Xiao , Qinming Wu , Chengtao Wang . Condensed Matter Chemistry in Catalysis by Zeolites[J]. Progress in Chemistry, 2023 , 35(6) : 886 -903 . DOI: 10.7536/PC221008
图3 以聚季铵盐PDADMAC为介孔模板合成的介孔Beta沸石与常规Beta沸石在苯与丙醇烷基化反应性能比较[51]Fig.3 Catalytic conversions (conv., solid) and selectivities (select., empty) in the alkylation of benzene with isopropanol vs. reaction time over mesoporous Beta (square) and conventional Beta (triangle) catalysts[51]. Copyright 2006, Wiley-VCH |
图4 介孔Y沸石和常规Y沸石的FCC性能比较[55]Fig.4 Catalyst evaluation of two FCC catalysts that were prepared and deactivated under the same conditions (788℃ in 100% steam for 8 h), one of which contained a conventional zeolite USY (—◆—), whereas the other contained a mesostructured zeolite USY (---▲---). The catalyst evaluation was performed in an ACE unit at 527℃ by using a VGO feedstock. The lines were fitted by a kinetic lump model[55]. Copyright 2012, RSC |
图6 不同的沸石催化剂在异丙苯催化裂化反应性能[58]Fig.6 Catalytic properties in catalytic cracking of cumene over (a) ZSM-5-TPA synthesized from tetrapropylammonium (TPA) template, (b) ZSM-5-OTF synthesized in the absence of organic template, and (c) mesoporous MFI-SDS synthesized in the presence of zeolite seeds but the absence of organic template[58]. Copyright 2016, Elsevier |
图15 不同催化剂在乙烷脱氢制乙烯反应中的性能[89]Fig.15 (A)Data showing the (a) ethane conversions and (b) ethene selectivities in a long-period ethane dehydrogenation (EDH) over the FeS-1-EDTA, PtSn/Al2O3, and Pt/Al2O3 catalysts. (c) Data characterizing the performance of the FeS-1-EDTA in propane dehydrogenation. (d) Data characterizing the performance of the PtSn/Al2O3 in propane dehydrogenation[89]. Copyright 2020, ACS |
图16 硼硅分子筛(BS-1)和S-1分子筛担载B物种(B/S-1)在丙烷有氧脱氢制丙烯反应中的性能[90]Fig.16 Dependences of propane conversion on reaction temperature over BS-1 and B/S-1; (B) dependences of olefin selectivity on propane conversion over BS-1; (C) the performances of BS-1 and B/S-1 before and after water treatment; (D) the durability data of BS-1[90]. Copyright 2021, Science |
图18 ZSM-5担载Pt催化剂的甲苯催化燃烧性能,其中R和O分别代表还原与氧化处理,Meso代表具有介孔结构[100]Fig.18 Catalytic performances in catalytic combustion of toluene over ZSM-5 supported Pt catalysts, where R and O stand for reduction and oxidation treatments for the catalysts, and meso means that the zeolite contains the mesoporosity[100]. Copyright 2015, Elsevier |
图24 在(a) Pt/Beta-Si, (b) Pt/Beta-SDS和(c) Pt/Beta-TEA催化剂上HCHO转化率与反应温度的关系,HCHO浓度80 ppm,O2 20%, 100 mL/min, 空速 60 000 mL/g·h, 相对湿度 50%, He为载气[108]Fig.24 Dependences of HCHO conversion on reaction temperature in HCHO oxidation over the (a) Pt/Beta-Si, (b) Pt/Beta-SDS, and (c) Pt/Beta-TEA catalysts under HCHO concentration of 80 ppm, O2 20%, rate of 100 mL/min, space velocity of 60000 mL/g·h, relative humidity of 50%, and He as the balance gas[108]. Copyright 2020, Elsevier |
图26 在AuPd@ZSM-5表面上修饰长链烷基,构建分子围栏,可以选择性地将所形成的双氧水保持于沸石晶体内部,与甲烷反应形成甲醇并穿透分子围栏,最终获得甲醇的高产率Fig.26 Hydrophobic zeolite modification of organic silane leads to construction of molecular fence, which is to enhance the reaction probability between methane and the generated hydrogen peroxide inside of zeolite crystals |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
( 谢在库, 等. 低碳烯烃催化技术基础. 北京: 中国石化出版社, 2013.).
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
/
| 〈 |
|
〉 |