

Atmospheric Chemistry and Measurement Research on Organic Nitrates
Received date: 2024-01-11
Revised date: 2024-03-23
Online published: 2024-07-01
Supported by
National Natural Science Foundation of China(42175151)
National Natural Science Foundation of China(U21A2028)
National Key Research & Development Program of China(2022YFC3701100)
Plan for Anhui Major Provincial Science & Technology Project(202203a07020003)
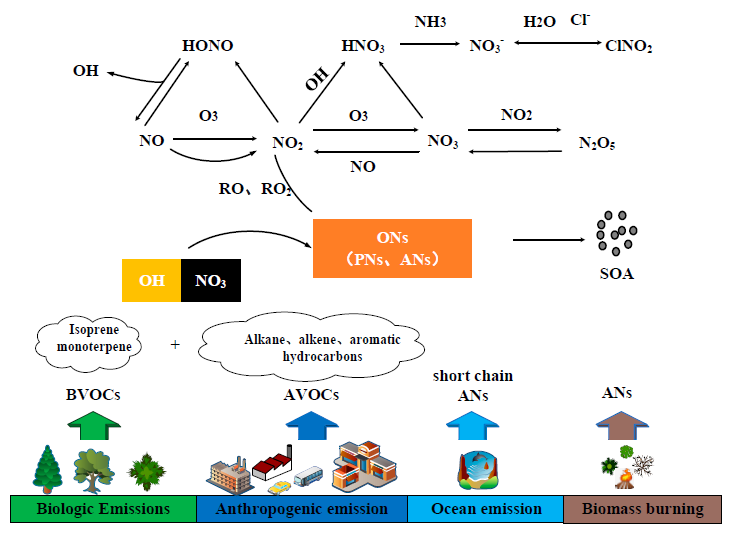
Organic nitrates(ONs)are an important nitrogen-containing organic compounds in the atmosphere.Atmospheric ONs are primarily produced by secondary photochemical reactions between atmospheric oxidants and volatile organic compounds in the presence of nitrogen oxides.Their generation and removal processes are closely related to the atmospheric nitrogen cycle,ozone,and the formation of secondary organic aerosols.Additionally,partial gas-phase ONs can enter the particle phase through gas-particle distribution or multiple oxidations,forming particle-phase ONs,which will promote the generation of PM2.5and further damage to human health.Currently,various chemical and spectroscopic measurement techniques have been successfully applied to measure the concentrations of single species and total ONs,and comprehensive field observations of various atmospheric components have been carried out in different regions to explore the mechanism of ONs generation involving nitrogen oxides,the impact of ONs on regional nitrogen chemistry,and the contribution of particulate ONs to secondary organic aerosols.This article discusses the important role of ONs in atmospheric chemistry by elucidating their mechanisms of generation and removal.It summarizes the measurement methods for ONs and highlights the characteristics and applications of different methods.The focus is placed on indoor smoke chamber researches related to ONs and field observation results in different regions.The observed data combined with atmospheric chemical models,further explain the atmospheric chemical effects of ONs.Additionally,it identifies some shortcomings in current researches,that need to be improved in subsequent researches 。
Contents
1 Introduction
2 Sources and sinks of atmospheric organic nitrates
3 Quantification methods of atmospheric organic nitrates
4 Researches on indoor smog chamber of organic nitrates
5 Field observations of organic nitrates
5.1 International field observations
5.2 Domestic field observations
6 Conclusion and outlook
Dou Shao , Min Qin , Wu Fang , Baobin Han , Jianye Xie , Xiadan Zhao , Zhitang Liao , Jiaqi Hu , Enbo Ren . Atmospheric Chemistry and Measurement Research on Organic Nitrates[J]. Progress in Chemistry, 2024 , 36(8) : 1145 -1156 . DOI: 10.7536/PC240111
表1 Different quantitative methods for ONs[42⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓~67]Table 1 The different quantitative methods for organic nitrates[42⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓~67] |
| Method | Characteristic | Application | Ref |
|---|---|---|---|
| GC/LC-MS | Low resolution, long offline analysis time | Quantitative specific ONs | 42⇓⇓⇓~46 |
| GC-ECD | Low detection limit and high sensitivity | Quantitative specific ONs | 47⇓~49 |
| CIMS | Soft ionization, fast response, high sensitivity, and strong selectivity | Quantitative specific ONs | 50⇓~52 |
| AMS | High resolution; uncertainty exists in the ratio method | Quantitative ∑ONs | 53⇓~55 |
| FTIR | Mature and simple, no pre-processing required; low resolution, high detection limit | Quantitative ∑ONs | 56,57 |
| TD | High resolution, sensitivity, requiring interference correction | Quantitative ∑ONs | 58⇓⇓⇓⇓⇓⇓⇓⇓~67 |
表3 PAN observation in typical areas of China[117,121,128,133,143]Table 3 PAN observations in some typical regions of China[117,121,128,133,143] |
| site | type | Time | Average concentration (ppbv) | Maximum concentration (ppbv) | Ref |
|---|---|---|---|---|---|
| Beijing | Rural | 2015.8~2019.2 | 0.94 | 2.41 | 121 |
| Urban | 2016.11~2017.1 | 1.2 | 7.1 | 117 | |
| The Pearl River Delta | Rural | 2006.7~2006.7 | 1.3 | 3.9 | 133 |
| Jinan, Shandong | Urban | 2016.4~2016.7 | 2.54 | 13.47 | 128 |
| Nam Co, Tibet Plateau | Remote | 2012.5~2012.7 | 0.44 | 0.99 | 143 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
(顾芳婷, 胡敏, 郑竞, 郭松. 化学进展, 2017, 29(9): 962.)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
(李伟, 黄山, 袁斌, 郭松, 邵敏. 中国环境科学, 2021, 41(7): 3017.)
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
(林川, 胡仁志, 谢品华, 吴盛阳, 童金钊, 李治艳, 王凤阳, 王怡慧. 光学学报, 2020, 40(12): 1201003.)
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
(邹宇, 邓雪娇, 李菲, 殷长秦. 环境科学, 2019, 40(4): 1634.)
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
(张剑波, 唐孝炎. 环境化学, 1994, 13(1): 30.)
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
/
| 〈 |
|
〉 |