

In Situ Transformation and Application of MXene
Received date: 2024-01-04
Revised date: 2024-03-14
Online published: 2024-07-01
Supported by
National Natural Science Foundation of China(62174086)
National Natural Science Foundation of China(62474096)
Outstanding Youth Foundation of Jiangsu Province(BK20240139)
Qinglan Project of Jiangsu Province of China, Postgraduate Research & Practice Innovation Program of Jiangsu Province(SJCX22_0253)
National Science and Technology Innovation Training Program(202310293156E)
MXene is a new class of two-dimensional transition metal carbides and nitrides which serves as a versatile and promising material with a wide range of applications in various fields.Layered MXene has abundant surface end-group functional groups(−F,−O and−OH)and a wide range of compatibility with second-phase materials,showing great potential in the construction of multi-functional,high-performance hybrid materials.Research has found that Ti3C2MXene nanosheets have a disadvantage of easy interlayer stacking during use,which is detrimental to ion/electron transport.The in-situ transformation of MXene provides a new approach to address this issue.During the in-situ transformation process of MXene materials,the loading of the second-phase material is controllable and can effectively suppress the interlayer stacking effect of MXene nanosheets.At the same time,by selecting and controlling the second-phase material,it is possible to achieve the directional construction of multifunctional,high-performance hybrid materials.The in-situ transformed hybrid materials can integrate the large specific surface area,metallic conductivity,high active sites of MXene,and the intrinsic properties of the selected second-phase material.Recently,there has been rapid development in the preparation and application of composite materials based on Ti3C2MXene derivatives,showcasing extensive research prospects in the fields of energy storage,catalysis,sensing,and more.Taking Ti3C2as an example,this article summarizes the preparation and transformation mechanisms of MXene-based in-situ converted hybrid materials(in-situ derived,metal ion hybridization,and MOF material hybridization).It also summarizes the applications of MXene hybrid materials in energy storage(lithium-sulfur batteries,supercapacitors,and hydrogen storage),sensors,and catalysis.The article points out the unresolved issues in MXene in-situ transformation research and outlines the future development directions for scientific research.It hopes to provide new research ideas for scholars in this field and contribute to the development of nanomaterials with functional properties 。
Contents
1 Introduction
2 In-situ transformation of MXene for hybrid materials
2.1 In-situ transformation of MXene
2.2 In-situ reaction of metal ions on the surface of MXene
2.3 Assembly of MOF with MXene
3 Application of MXene derived nanocomposites
3.1 Energy storage
3.2 Sensor
3.3 Catalysis
4 Summary and outlook
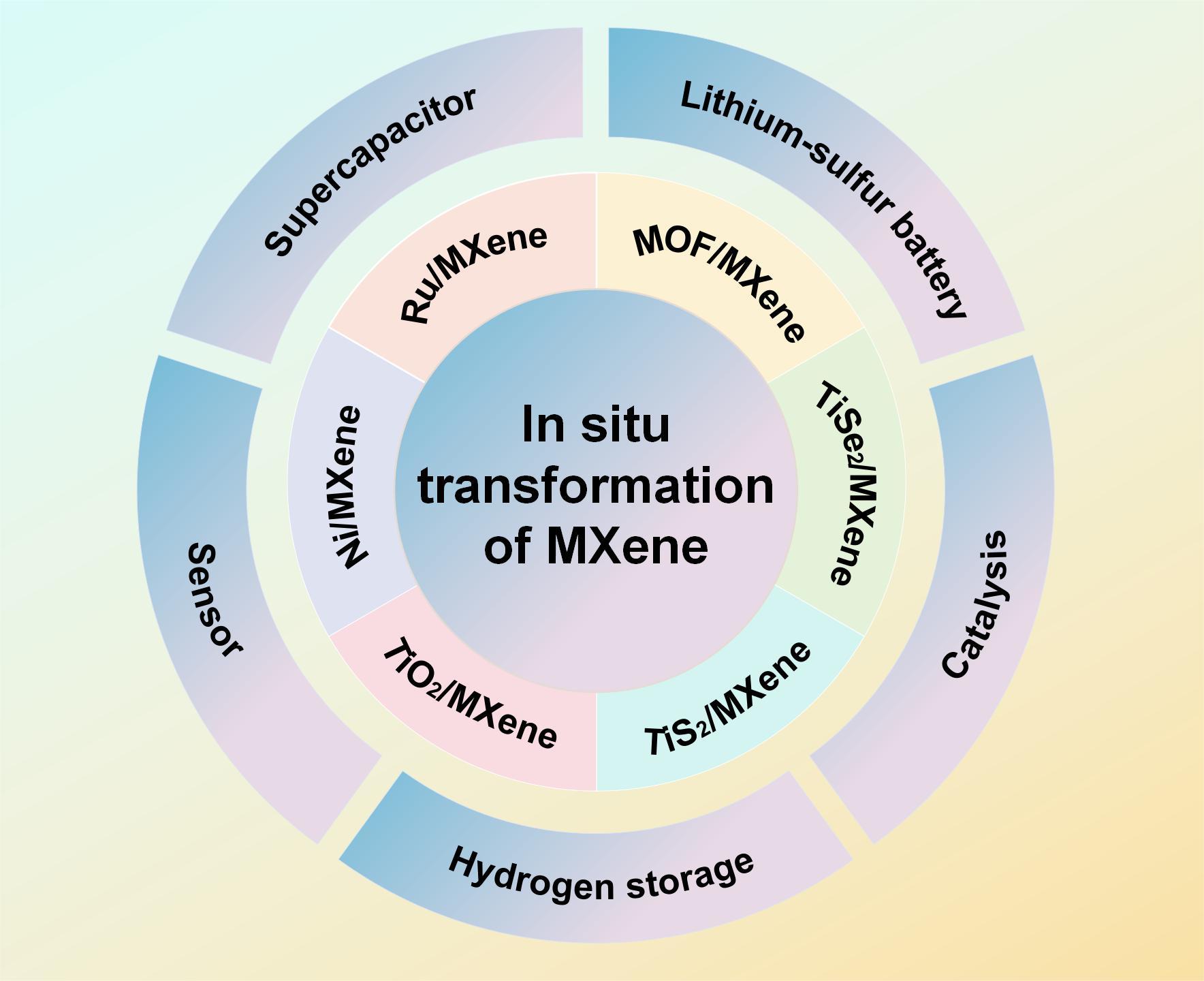
Key words: MXene; in-situ transformation; nanomaterials; catalysis; energy storage; sensor
Yunshuo Zhang , Feifei Lin , Yuzhe Chen , Ning Ding , Yulan Wei , Weiwei Zhao . In Situ Transformation and Application of MXene[J]. Progress in Chemistry, 2024 , 36(8) : 1174 -1185 . DOI: 10.7536/PC231215
图1 (a)刻蚀Ti3C2示意图; (b)手风琴状Ti3C2的SEM图像[29]; (c)零维(米壳状)TiO2与Ti3C2形成的复合材料的SEM图像[23]; (d)一维TiO2与Ti3C2形成的复合材料的SEM图像[28]; (e)二维TiO2与Ti3C2形成的复合材料的SEM图像[30]Fig. 1 (a)Schematic diagram of etching Ti3C2; (b)SEM images of accordion-like Ti3C2[29]; (c)SEM image of 0D (rice crust) TiO2 and Ti3C2 composite material[23]; (d)SEM image of 1D TiO2 and Ti3C2 composite material[28]; (e)SEM image of 2D TiO2 and Ti3C2 composite material[30] |
图5 (a)LiPSs在TiO2-Ti3C2异质结构上的俘获和转化过程示意图;(b)Ti3C2 -GN夹层的Li-S电池循环性能;(c~e)不同异质结构电极上Li2S8溶液在2.02 V下的恒电位放电曲线[63]Fig. 5 (a) Schematic illustration of LiPSs trapping and conversion process on the TiO2-Ti3C heterostructures; (b) Cycling performance of Li-S cells with Ti3C2 -GN interlayer; (c~e) Potentiostatic discharge profiles of Li2S8 solution at 2.02 V on the electrodes containing different heterostructures[63] |
图6 (a)CeF3/Ti3C2制备及应用示意图;储氢性能表征:(b)TPD曲线, (c)体积释放曲线, (d, e)等温脱氢曲线, (f)NaAlH4+10CeF3/Ti3C2复合材料的循环脱氢实验(插图为相应的氢容量保留量)[78]Fig. 6 (a) Scheme of preparation and application of CeF3/Ti3C2;Hydrogen storage property characterization: (b)TPD curves, (c)Volumetric release curves, (d, e)Isothermal dehydrogenation curves, (f) The cycling dehydrogenation tests of the NaAlH4+10CeF3/Ti3C2 composite(the inset is the corresponding hydrogen capacity retention)[78] |
图7 (a)Co-TCPP(Fe)、Ti3C2及Co-TCPP(Fe)/Ti3C2的合成工艺示意图;(b)10 ppm NO暴露于原始Co-TCPP(Fe)、Ti3C2、TCPP(Fe)/Ti3C2-20、TCPP(Fe)/Ti3C2-20和Co-TCPP(Fe)/ Ti3C2-20后的典型响应曲线; (c)10 ppm NO暴露于Co-TCPP (Fe)/Ti3C2后的典型响应曲线; (d)室温下Co-TCPP(Fe)/Ti3C2-20传感器对10 ppm NO的实时响应-恢复曲线; (e)室温下不同相对湿度(0% ~ 70%),传感器对10 ppm NO的响应; (f)传感器在室温下暴露于10 ppm NO时的长期稳定性; (g)传感器对浓度为10 ppm和20 ppm的各种气体的选择性[86]Fig. 7 (a) Synthesis process of Co-TCPP(Fe),Ti3C2,and Co-TCPP(Fe)/Ti3C2; (b) Typical response curves after 10 ppm NO exposure to pristine Co-TCPP(Fe),Ti3C2,TCPP(Fe)/ Ti3C2-20,TCPP(Fe)/Ti3C2-20 and Co-TCPP(Fe)/Ti3C2-20; (c) Typical response curves after 10 ppm NO exposure to Co-TCPP(Fe)/Ti3C2; (d) Real-time response-recovery curve of the Co-TCPP(Fe)/Ti3C2-20 based sensor toward 10 ppm NO at room temperature; (e) The response of the sensor toward 10 ppm NO at different relative humidities ranging from 0% to 70% at room temperature; (f) Long-term stability of the sensor exposed toward 10 ppm NO at room temperature; (g) The selectivity of the sensor to various gases at concentrations of 10 ppm and 20 ppm [86] |
图8 (a)CoMoS2/Ti3C2结构示意图; (b)Ti3C2 MXene、CoMoS2及CoMoS2/Ti3C2合成原理图; CoMoS2/Ti3C2的加氢脱硫催化性能:Ti3C2 MXene、CoMoS2及CoMoS2/Ti3C2-200的(c)氮气吸附-解吸等温线和(d)孔径分布图[16]Fig. 8 (a)Schematic of the structure of CoMoS2/Ti3C2; (b)Schematic of the synthesis of Ti3C2 MXene, CoMoS2, and CoMoS2/Ti3C2; Catalytic performance of as-prepared samples:(c)Nitrogen adsorption-desorption isotherms and (d)pore size distribution plots of Ti3C2 MXene, CoMoS2 and CoMoS2/Ti3C2-200[16] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
/
| 〈 |
|
〉 |