

Synthesis and Cancer Biomedical Applications of Dendrimer-Based Fluorescence Imaging Agents
Received date: 2024-01-02
Revised date: 2024-02-12
Online published: 2024-02-26
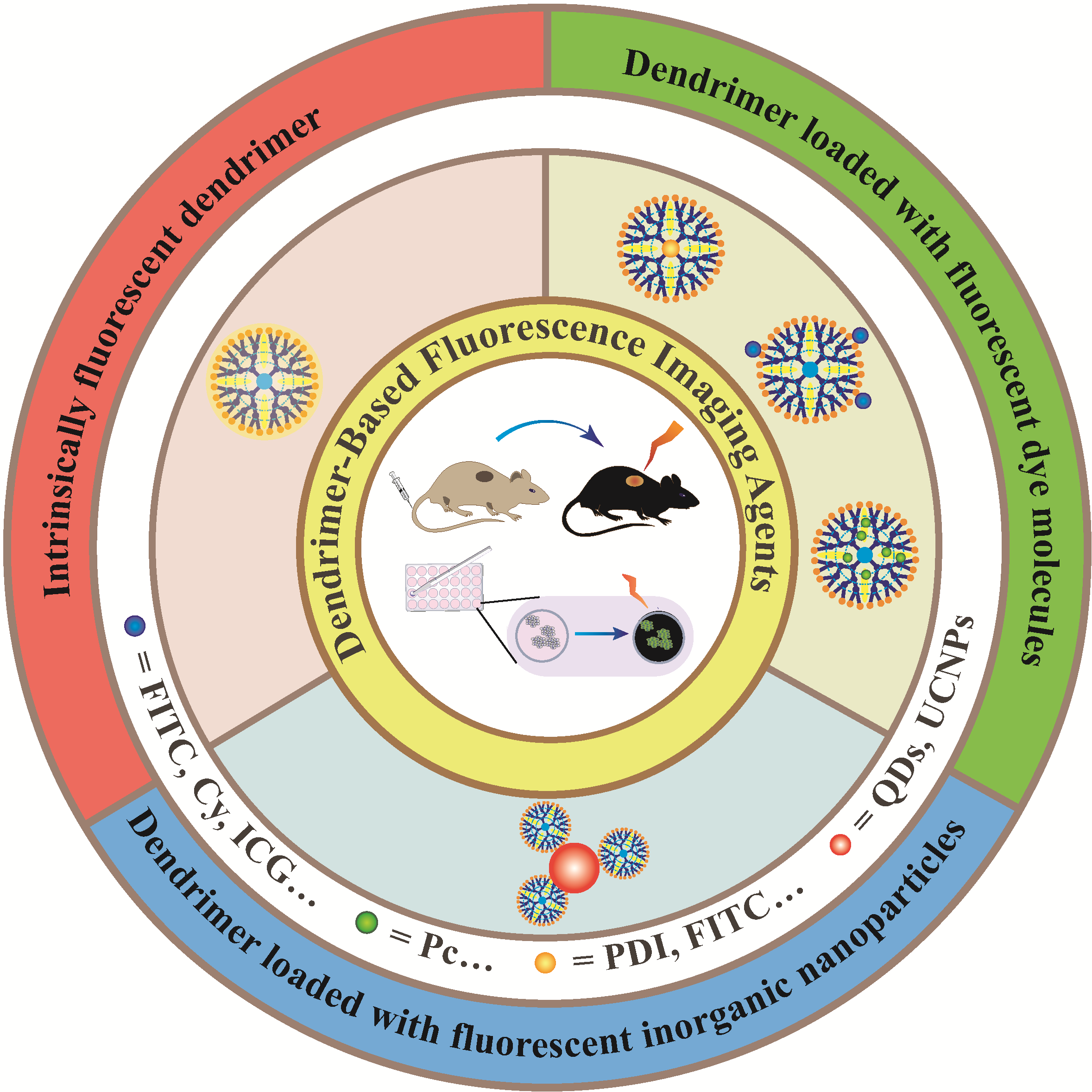
as a molecular imaging technique with high sensitivity and high spatial resolution,fluorescence imaging is widely used in cancer diagnosis and therapy.However,commonly used fluorescence imaging agents,such as small-molecule fluorescent dyes and fluorescent inorganic nanoparticles,have defects such as poor photostability,rapid metabolism in vivo,and low accumulation at lesion sites,which limit their application in the field of cancer fluorescence imaging.in recent years,the appearance of dendrimer has provided a new strategy for the development of nano-scale fluorescence imaging agents.A dendrimer is composed of three parts,including a central core,internal repeating units and abundant terminal functional groups.the excellent structure of dendrimer enables it to load small-molecule fluorescent dyes or fluorescent inorganic nanoparticles to achieve early fluorescence monitoring of cancer and evaluate its distribution and metabolism in vivo.Additionally,some amino-terminated dendrimers can be used to monitor their uptake by cancer cells through their intrinsic fluorescence.the introduction of dendrimer greatly improves the water solubility and biocompatibility of fluorescent dyes and fluorescent inorganic nanoparticles,and the surface functionalization of dendrimer could achieve their tissue-specific delivery.Most importantly,the protection of dendrimer can greatly avoid fluorescence quenching and achieve long-time fluorescence imaging.Therefore,this review mainly describes various kinds of dendrimer-based fluorescence imaging agents,summarizes their synthesis methods and their applications in cancer fluorescence imaging,and prospects for their future development。
Contents
1 Introduction
2 Functionalized dendrimer with fluorescence property
2.1 Intrinsically fluorescent dendrimer
2.2 Dendrimer loaded with fluorescent dye molecules
2.3 Dendrimer loaded with fluorescent inorganic nanoparticles
3 Conclusion and outlook
Linjie Yue , Lingxiu He , Na Liu , Risong Pan , Jingyi Zhu . Synthesis and Cancer Biomedical Applications of Dendrimer-Based Fluorescence Imaging Agents[J]. Progress in Chemistry, 2024 , 36(8) : 1186 -1199 . DOI: 10.7536/PC240101
图3 (a)ZIPP-Apt:DOX/siHSPs的合成示意图;(b)荷瘤鼠经尾静脉注射ZIPP(Cy5.5)-Apt或ZIPP(Cy5.5)-CRO后不同时间点下的荧光成像图及(c)注射后48 h的离体器官及肿瘤的荧光成像图[36]Fig. 3 (a)Schematic synthesis process of ZIPP-Apt: DOX/siHSPs; (b) fluorescence images of tumor-bearing mice at different time points after intravenous injection of ZIPP(Cy5.5)-Apt or ZIPP(Cy5.5)-CRO, and (c) fluorescence images of ex vivo organs and tumors at 48 h after injection[36] |
图5 生物素靶向PDI-PLL-G5用于体内异种移植瘤成像。(a)静脉注射各材料后4T1荷瘤鼠的全身光学成像;(b)不同成像时间点下的肿瘤荧光信号定量分析;(c)不同成像时间点下的肿瘤与正常组织的TNR[35]Fig. 5 Biotin-targeted PDI-PLL-G5 for in vivo xenograft tumor imaging. (a) Whole-body optical imaging of 4T1 tumor-bearing mice after intravenous injection of different materials. (b) Quantitative fluorescence signals in the tumor at different imaging times. (c) TNR of tumor and normal tissue at different imaging times[35] |
图6 (a)T-DPHDs的合成示意图;(b)4T1荷瘤鼠的体内荧光图像和原发肿瘤的离体荧光图像;(c)注射后24 h肺部的离体图像(白色箭头表示转移灶)[51]Fig. 6 (a) Schematic illustration of the synthesis of the T-DPHDs. (b) In vivo fluorescence images of 4T1 tumor-bearing mice and ex vivo fluorescence images of primary tumors. (c) Ex vivo images of the lung at 24 h post-injection. The white arrows indicate the metastatic foci[51] |
图8 (a)FA和G4.5包裹混合镧系(Dy/Er/Tb)氧化物纳米颗粒的简易合成。(b)FA@Dy, Er, Tb oxide NPs(1)和G4.5@Dy, Er, Tb oxide NPs(2)孵育HeLa细胞24 h后的FL成像图[47]Fig. 8 (a) Facile synthesis of mixed lanthanide (Dy/Er/Tb) oxide nanoparticles coated by FA and G4.5. (b) FL imaging of HeLa cells after 24 h incubation with FA@Dy, Er, Tb oxide NPs (1) and G4.5@Dy, Er, Tb oxide NPs (2)[47] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
/
| 〈 |
|
〉 |