

Principle and Application of Electro-Fermentation Technology for Enhancing the Resource Utilization of Organic Waste
Received date: 2024-01-08
Revised date: 2024-06-05
Online published: 2024-07-01
Supported by
National Natural Science Foundation of China(22206103)
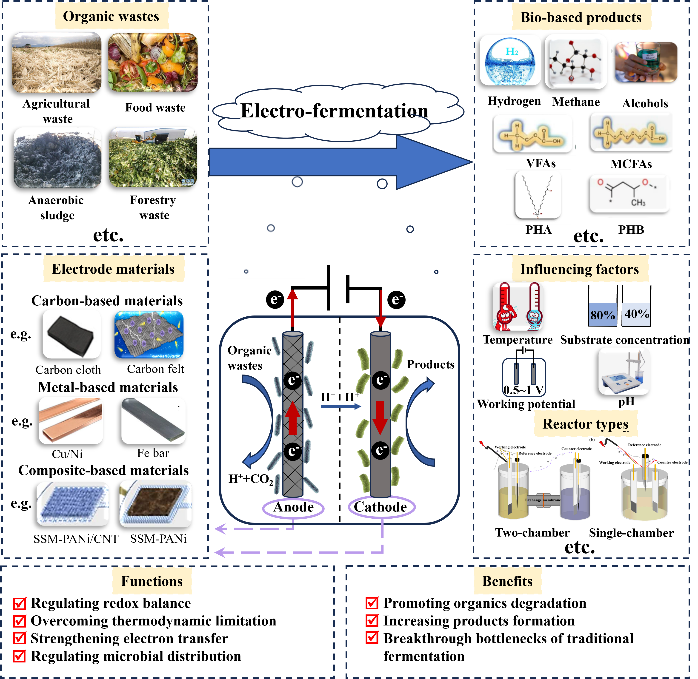
the increasing production of organic waste poses a challenge for waste treatment and disposal.Due to the richness of nutrients such as polysaccharides,proteins and minerals,the Bio-resourcing of organic waste has attracted much attention.Currently,the traditional anaerobic fermentation system for organic waste treatment has problems such as long fermentation period,low product yield,poor product selectivity,and low degradation rate of organic waste.the bio-electro-fermentation technology formed by applying electrochemical system to the traditional anaerobic fermentation system can regulate the redox balance of microbial reaction by electrochemical methods,overcome the thermodynamic limitations of traditional fermentation,strengthen the microbial electron transfer,promote the degradation of organic wastes and the generation of resource products,and achieve the high efficiency of organic waste resourcing.in this paper,we systematically investigate the basic principles of electro-fermentation technology to promote organic waste resources,review the electrode materials,microorganisms,reactor structure,and methods to enhance the operation of the system,introduce the current research status of electro-fermentation technology to strengthen the generation of organic waste resource products(including methane,hydrogen,alcohols,short-chain fatty acids,medium-chain fatty acids,polyhydroxybutyrate,polyhydroxyalkanoate,etc.),summarize and look forward to the prospects and challenges for the development of electro-fermentation technology In the application of organic waste resource utilization。
Contents
1 Introduction
2 Principle of electro-fermentation
2.1 Principle of cathodic electro-fermentation
2.2 Principle of anodic electro-fermentation
2.3 Extracellular electron transfer
3 Electrode materials used in electro-fermentation
3.1 Carbon-based materials
3.2 Metal-based materials
3.3 Composite-based materials
4 Microorganisms in electro-fermentation systems
5 Single and double chamber electro-fermentation systems
6 Methods for improving the operation of electro-fermentation systems
6.1 Condition optimization
6.2 Conductive medium
6.3 Bioaugmentation
7 Products of electro-fermentation
7.1 Methane
7.2 Hydrogen
7.3 Alcohols
7.4 Volatile fatty acids
7.5 High-value chemicals
8 Conclusions and Perspectives
Xiaoyan Sun , Yanan Yin , Hui Chen , Lei Zhao , Cheng Wang , Jianlong Wang . Principle and Application of Electro-Fermentation Technology for Enhancing the Resource Utilization of Organic Waste[J]. Progress in Chemistry, 2024 , 36(8) : 1200 -1216 . DOI: 10.7536/PC240104
图1 阴极电发酵的潜在机制:(a)强化电子传递,(b)氧化还原电位调控,(c)互营协作(黄色为微生物,橙色为电活性微生物,MedOx为氧化介质,MedRed为还原介质)Fig. 1 Potential mechanism of cathodic electro-fermentation (a) strengthening of electron transfer, (b) regulation of redox potential, (c) mutual cooperation (yellow represents microorganisms, orange represents electroactive microorganisms, MedOx is the oxidizing medium, and MedRed is the reducing medium) |
图2 阳极电发酵的潜在机制:(a)强化电子传递,(b)氧化还原电位调控,(c)互营协作(黄色为微生物,橙色为电活性微生物,MedOx为氧化介质,MedRed为还原介质)Fig. 2 Potential mechanisms of anodic electro-fermentation. (a) strengthening of electron transfer, (b) regulation of oxidation-reduction potential, (c) mutual collaboration (Yellow represents microorganisms, orange represents electroactive microorganisms, MedOx is the Oxidizing Medium, and MedRed is the reducing medium) |
表1 Extracellular Electron Transfer Pathway between MicroorganismsTable 1 Extracellular electron transfer pathways between microorganisms |
| Electronic transfer type | Electron transfer mechanism | Notes |
|---|---|---|
| Direct electron transfer | Direct contact route | Microorganisms form aggregates or directly contact solid surfaces, and electron transfer between microorganisms or between microorganisms and electrodes is carried out through the pigment proteins on the surface of microorganisms |
| Nanowire pathway | Microbial conductive pili mediate electron transfer between microorganisms | |
| Indirect electron transfer | Electron shuttle mediated | Redox substances present in the environment or secreted by microorganisms themselves, such as H2, formic acid, phenazine, humic acid, riboflavin, and anthraquinone, mediate electron transfer |
| Conductive materials mediate | Electron transfer mediated by non-biological conductive materials such as carbon, metal elements, metal oxides, and alloys |
表2 Main electroactive microorganisms in electrofermentation systemTable 2 Main electroactive microorganisms in the electro-fermentation system |
| Strain | Genus | Example |
|---|---|---|
| Geobacter sulfurreducens | Geobacteraceae | Acetate as an electron donor for G. sulfurreducens to produce methane[93] |
| Geobacter metallireducens | Geobacteraceae | G. metallireducens acts as an electron donor, transferring electrons to Rhodopseudomonas palustris to achieve CO2 fixation[94] |
| Shewanella oneidensis MR-1 | Shewanellaceae | In microbial fuel cells, when lactic acid is used as a carbon source, it can be metabolized by S. oneidensis MR-1 to form DADH, and the released electrons can be transferred to the anode to form a current[95] |
| Shewanella loihica | Shewanellaceae | Shewanella loihica PV-4 can reduce fumarate by using the electrode as the sole electron donor[96] |
| Thermincola potens | Peptococcaceae | In microbial fuel cells, proteins on the surface of Thermincola potens JR cells transfer electrons to the electrode to reduce Fe (Ⅲ)[97] |
| Thermincola ferriacetica | Peptococcaceae | In microbial fuel cells, T. ferricetica can directly transfer electrons from acetic acid to the anode working electrode to generate current[90] |
| Calditerrivibrio nitroreducens | Desulfobulbaceae | In microbial fuel cells, C. nitroreducens can utilize nitrate as an electron acceptor, and the system stably generates a current density of 272 mW·m-2[98] |
| Desulfobulbus propionicus | Desulfobulbaceae | Desulfobulbus propionicus uses pyruvate as an electron donor to transfer electrons to the anode working electrode and convert them into acetate[99] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
(冯应鸿. 大连理工大学硕士学位论文, 2014 )
|
| [5] |
(王华金, 朱能武, 李冲, 程丹, 吴平霄. 农业环境科学学报, 2012, 31(7): 14317.)
|
| [6] |
(邹勇进, 孙立贤, 徐芬, 杨黎妮. 高等学校化学学报, 2007, 28(3): 510.)
|
| [7] |
(马晨, 周顺桂, 庄莉. 生态学报, 2011, 31(7): 2008.)
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
(王艺碹. 中国科学技术大学博士学位论文, 2020.)
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
(张林防. 重庆大学博士学位论文, 2020.)
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
| [173] |
Alberto García Mogollón C, Carlos Quintero Díaz J,
|
| [174] |
|
| [175] |
|
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
|
| [180] |
|
| [181] |
|
| [182] |
|
| [183] |
(陈哲柯. 湖南大学硕士学位论文, 2018.)
|
| [184] |
(吴清莲. 哈尔滨工业大学博士学位论文, 2019.)
|
| [185] |
|
| [186] |
Van Eerten-Jansen M C A A,
|
| [187] |
|
| [188] |
|
| [189] |
|
| [190] |
|
| [191] |
|
| [192] |
|
| [193] |
|
| [194] |
|
/
| 〈 |
|
〉 |