

Application of Metal-Organic Frameworks for Battery Separators
Received date: 2024-01-11
Revised date: 2024-04-08
Online published: 2024-07-01
Supported by
National Natural Science Foundation of China(12372245)
Postgraduate Scientific Research Innovation Project of Hunan Province(CX20240908)
Natural Science Foundation of Hunan Province(2024JJ7164)
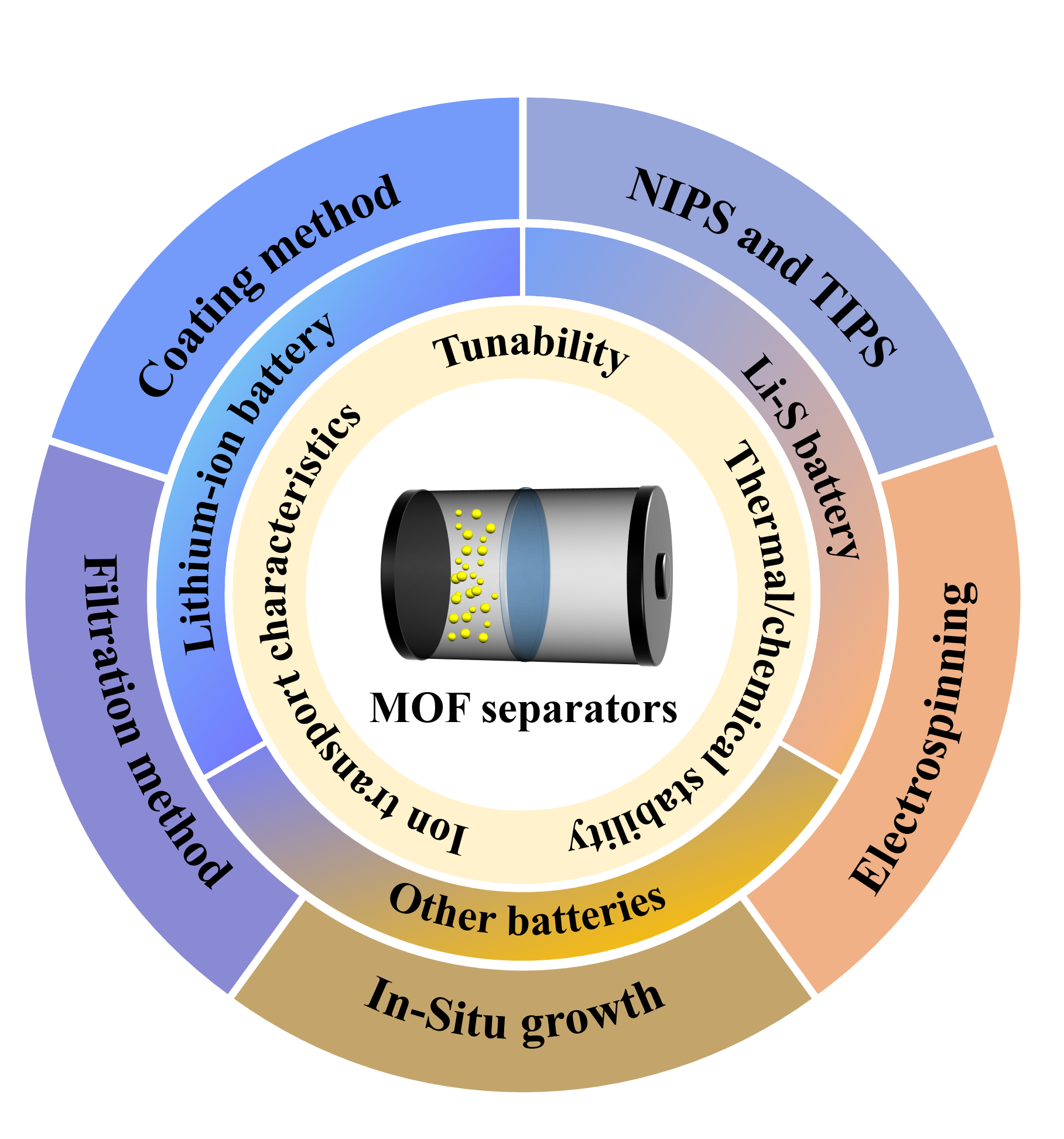
With the rapid development of the new energy industry,research on different kinds of high-performance batteries has become a hot topic nowadays.As one of the important components of batteries,the separator can effectively prevent direct contact between positive and negative electrodes of batteries and provide favorable channels for ion transport.However,traditional polymer battery separators usually have problems such As insufficient thermal stability,poor ion transport capacity,and poor electrolyte wettability.As a new type of porous crystalline material,metal-organic frameworks(MOFs)have become the current research hotspot for high-performance battery separators due to their high porosity,high specific surface area and excellent thermal stability.in this paper,the applications of various MOFs or MOFs-based materials in battery separators are reviewed,and the advantages and disadvantages of MOFs-based battery separators are comprehensively discussed.Finally,the urgent problems to be solved in the field of MOFs-based battery separators and the development prospects of MOFs in battery separators are presented。
Contents
1 Introduction
2 Lithium-ion battery separators based on MOFs
2.1 Original MOFs based separators
2.2 MOFs composites-based separators
2.3 MOFs derivatives-based separators
3 Lithium-sulfur battery separator
3.1 Original MOFs based separators
3.2 MOFs composites-based separators
3.3 MOFs derivatives-based separators
4 Other types of battery separator based on MOFs
5 Conclusion and outlook
Key words: metal-organic frameworks (MOFs); battery separator; syntheses
Pengcheng Xiao , Saiqun Nie , Mingliang Luo , Jiayao Chen , Fuli Luo , Tian Zhao , Yue-Jun Liu . Application of Metal-Organic Frameworks for Battery Separators[J]. Progress in Chemistry, 2024 , 36(8) : 1217 -1236 . DOI: 10.7536/PC240110
表1 Performance table of MOF-based lithium-ion battery separatorTable 1 The performances of main MOF-based lithium-ion battery separators |
| Material | Cycling performance (mAh·g−1) | Rate performance (mAh·g−1) | Ionic conductivity (mS·cm−1) | Li+ transference number tLi+ | ref | |
|---|---|---|---|---|---|---|
| ZIF-67/PP | 104.4 (100th/1 C) | 44 (5 C) | 1.64 | - | 61 | |
| UIOSOL@PP | 155 (600th/1 C) | 125 (5 C) | 1.09 | 0.82 | 89 | |
| ZIF-67-H2O/PP | 93.92 (100th/1 C) | - | 0.62 | - | 62 | |
| ZIF-67-CH3OH/PP | 104.7 (100th/1 C) | - | 0.78 | - | 62 | |
| ZIF-8-0.5/CNFs | - | - | 0.41 | 0.41 | 70 | |
| ZIF-8-1/CNF | - | - | 0.45 | 0.45 | 70 | |
| ZIF-8-2/CNFs | 112 (100th/1 C) | 82 (8 C) | 1.41 | 0.50 | 70 | |
| BC/ZIF-8 | 133 (100th/0.5 C) | 102.3 (3 C) | 1.12 | - | 73 | |
| ZIF-8@BC/ANFs | 140.3 (100th/0.5 C) | 114.2 (3 C) | 1.60 | - | 75 | |
| LC-UiO-66/PVA | - | - | 2.90 | 0.59 | 66 | |
| LP-UiO-66/PVA | - | - | 1.90 | 0.79 | 66 | |
| CA/PU10-UiO-67 | - | - | 1.19 | 0.59 | 78 | |
| CA/PU30-UiO-67 | 112 (900th/1 C) | - | 0.48 | 0.71 | 78 | |
| PLA@PDA-ZIF-8 | 138.3 (200th/1 C) | - | 1.99 | - | 51 | |
| BC/ZIF-67 | - | - | 0.837 | - | 74 | |
| UiO-66-NH-(CH2)3 —SO3-/PVDF-HFP@GF | 76 (3000th/5 C) | 45 (20 C) | 0.67 | 0.74 | 90 | |
| MIL-125/PVDF-HFP | - | 73 (2 C) | 2.20 | - | 40 | |
| MOF-808/PVDF-HFP | - | 64 (2 C) | 2.40 | - | 40 | |
| UiO-66-NH2 | /PVDF-HFP | 80 (100th/1 C) | 85 (2 C) | 3.10 | - | 40 |
| PVDF/MOF-808 | - | 50 (2 C) | 3.80 | - | 79 | |
| UiO-66-S@OPBI | 94.9 (200th/0.5 C) | 97.9 (5 C) | 1.21 | 0.53 | 88 | |
| UiO-66-S-Li@OPBI | 107 (200th/0.5 C) | 103.1 (5 C) | 1.46 | 0.73 | 88 | |
| GU@PAN | 125 (200th/1 C) | 58 (10 C) | - | 0.603 | 67 | |
| PVDF-HKUST-1 | 121.5 (250th/1 C) | 143.7 (2 C) | 0.61 | - | 81 | |
表2 Performance table of intrinsic MOF-based lithium-sulfur battery separatorTable 2 The performance of original MOFs-based lithium-sulfur battery separator |
| Material | Initial discharge capacity (mAh·g-1) | Cycling performance (mAh·g-1) | Sulfur loading (mg·cm−2) | ref |
|---|---|---|---|---|
| Mn-BTC | 1450 (0.1 C) | 1100 (800th/0.1 C) | - | 107 |
| Y-FZTB | 1101 (0.25 C) | 557 (300th/0.25 C) | 1.0 | 39 |
| ZIF-67 | 1025(0.25 C) | 452 (300th/0.25 C) | 1.0 | 39 |
| ZIF-8 | 989(0.25 C) | 403 (300th/0.25 C) | 1.0 | 39 |
| HKUST-1 | 1032(0.25 C) | 197 (300th/0.25 C) | 1.0 | 39 |
| MIL-125(Ti) | 1218.3(0.2 C) | 726 (200th/0.2 C) | 2.0 | 108 |
| UiO-66 | 1032 (0.5 C) | 586 (500th/0.5 C) | 1.5 | 110 |
| Ni3(HITP)2 | 1244 (0.2 C) | 1139 (100th/0.2 C) | 3.5 | 109 |
| ZIF-8 | 945 (0.2 C) | 581 (100th/0.2 C) | 3.5 | 109 |
| Cu2(CuTCPP) | 1200 (0.2 C) | 1020 (100th/0.2 C) | 2.0 | 111 |
| FJU-88 | 955.7 (0.5 C) | - | 1.0 | 38 |
| FJU-90 | 1382.3 (0.2 C) | 826.5 (500th/1 C) | 1.0 | 38 |
| Ce-UiO-67 | 1288 (0.2 C) | 919 (500th/1 C) | 2.0/7.0 | 112 |
表3 Performance table of MOF-based composite lithium-sulfur battery separatorTable 3 The performance of MOF composites-based lithium-sulfur battery separator |
| Material | Initial discharge capacity (mAh·g-1) | Cycling per formance (mAh·g-1) | Rate performance (mAh·g-1) | ref |
|---|---|---|---|---|
| PSS@HKUST-1/Celgard | 1278 (0.5 C) | 775 (500th/0.5 C) | 335 (5 C) | 113 |
| CNT@ZIF-8 | 1588.4 (0.2 C) | 870 (100th/0.2 C) | 583.2 (2 C) | 115 |
| Co/NCNS/CNT | 1253 (0.1 C) | 522 (500th/1 C) | 849 (2 C) | 116 |
| B/2D MOF-Co | 1138 (0.1 C) | 580 (500th/0.5 C) | 552 (5 C) | 117 |
| Co@CoO@N-C/rGO | 1385 (0.1 C) | 555 (500th/1 C) | 555 (2 C) | 124 |
| Ni-C(B) | 1413.7 (0.05 C) | 600 (300th/0.5 C) | 556 (1 C) | 125 |
| Ni-C(T) | 1230 (0.05 C) | 472 (300th/0.5 C) | 415 (1 C) | 125 |
| CoSe2@C-N/CNT | 1224 (0.5 C) | 761 (300th/1 C) | 798 (3 C) | 126 |
| ZIF-67@PCF | 1221 (0.2 C) | 812 (500th/1 C) | 661 (5 C) | 120 |
| PCN@CNT | 1157 (0.1 C) | 545 (500th/1 C) | 696 (2 C) | 127 |
| MXene/PP /Cu-TCPP | 1275.5 (0.1 C) | 687 (100th/0.5 C) | 504 (1 C) | 128 |
| PPW/UiO-66 @BP | 1344 (0.1 C) | 759 (1000th/0.5 C) | 525 (4 C) | 118 |
| F-ZIF-67-PMIA | 1267.5 (0.5 C) | 689 (500th/0.5 C) | 916 (1 C) | 129 |
| F-Cu-BTC-PMIA | 1272.2 (0.5 C) | 754 (500th/0.5 C) | 969 (1 C) | 129 |
| ZIF-L(Co)-PMIA | 1391.2 (0.2 C) | 961 (350th/0.2 C) | 774 (2 C) | 119 |
| MOF/Nafion | - | 534.5(300th/0.5 C) | - | 114 |
| ZIF-67/PW10V2 | 1637.6 (0.2 C) | 1054.6(120th/0.5 C) | 802.7(2 C) | 122 |
| Cu-MOF/MXene | 1203 (0.5 C) | 1100 (300th/1 C) | 1081(2 C) | 123 |
| Fe-MOF/MXene | 1297 (0.5 C) | 1131 (300th/1 C) | 1169(2 C) | 123 |
表4 Performance table of MOFs derivative-based lithium-sulfur battery separatorTable 4 Performance of MOF derivatives-based lithium-sulfur battery separator |
| Material | Initial discharge capacity (mAh·g−1) | Cycling performance (mAh·g−1) | Rate performance (mAh·g−1) | ref | |
|---|---|---|---|---|---|
| Ce-UiO-66-NH2 | 1366 (0.2 C) | 627 (300th/2 C) | 653.3 (2 C) | 136 | |
| UiO-66-NH2@ graphene | 1598 (0.2 C) | 800 (100th/1 C) | 705 (3 C) | 137 | |
| UiO-66(SO3Li)4 | 1493.3 (0.1 C) | 457 (1000th/1 C) | 730.1 (2 C) | 139 | |
| aMIL-88B | 1508 (0.1 C) | 740 (500th/1 C) | 610 (5 C) | 141 | |
| In/Zr-BTB@PP | 1067.7 (0.2 C) | 679 (500th/2 C) | 637.7 (5 C) | 142 | |
| NiCo2S4@C | 1383 (0.1 C) | 700 (200th/2 C) | 850 (2 C) | 143 | |
| Co/CNT@DCNS | 1235 (0.2 C) | 691 (500th/0.5 C) | 870 (2 C) | 144 | |
| Co8S9 | 1385 (0.1 C) | 1190 (200th/0.1 C) | 428 (2 C) | 147 | |
| CeO2-C | 1240 (0.1 C) | 520 (500th/0.5 C) | 531 (1 C) | 145 | |
| N-C-1000 | 1200 (0.2 C) | / | 531 (1 C) | 148 | |
| F-N-C-1000 | 1250 (0.2 C) | 640 (500th/2 C) | 500 (5 C) | 148 | |
| OMNC | 1257.1 (0.5 C) | 736 (300th/0.5 C) | / | 149 | |
| N,S-Mo2C/C-ACF | 1200 (0.2 C) | 432 (600th/1 C) | 630 (5 C) | 150 | |
| MnSN-C@CNT | 1181 (0.1 C) | 500 (500th/0.5 C) | 627 (1 C) | 146 | |
| MIL1000 | 1244.6 (0.1 C) | 570 (500th/1 C) | 739.1 (2 C) | 151 | |
| Co3O4/Zno/PE | 875.5 (0.5 C) | 500 (400th/0.5 C) | 666.7 (1 C) | 152 | |
| MMMS | 1069 (0.1 C) | 580 (500th/0.5 C | 552 (5 C) | 138 | |
| Ni-Co MOF@PAN | 1560 (0.1 C) | 242 (2000th/2 C) | 532 (10 C) | 155 | |
| Fe SAs-NC/PP | 1052 (0.1 C) | 789 (200th/0.2 C) | 420 (5 C) | 157 | |
| Fe-ZIF-8/PP | 863 (0.5 C) | 555 (100th/0.1 C | 746 (3 C) | 158 | |
| Ti3C2Tx/Ni-Co MOF@PP | 1260 (0.2 C) | 91% (350th/0.5 C) | 660 (2 C) | 156 | |
| S-UiO-66-(OH)2/PP | 1235 (0.2 C) | 733.45 (500th/1 C) | 623 (5 C) | 140 | |
| D-UiO-66-(OH)2/PP | 806.2 (0.2 C) | 750.7(100th/0.2 C) | / | 140 | |
| S-MIL-101 | 1444.6 (0.2 C) | 990.4(100th/0.2 C) | 803.5 (3 C) | 159 | |
表5 Comparison of advantages and disadvantages of MOFs-based battery separator preparation methodsTable 5 Comparison of advantages and disadvantages of various preparation methods for the MOFs-based separators |
| 制备方法 | 优势 | 劣势 |
|---|---|---|
| 涂布法 | 简单高效,易于大规模生产 | 需要考虑MOF颗粒与基底材料之间的结合力和界面相容性 |
| 真空抽滤法 | 适用于实验室小规模制备,简单高效 | 仅适用于纤维素基质或少数聚合物基质,且MOF颗粒必须均匀地分散在基质悬浮液中 |
| 原位生长法 | 制备过程简单,MOF颗粒分散均匀,不易从基底材料上脱落 | 仅适用于可在室温下制备的MOF,如ZIF-8、ZIF-67、UiO-66等 |
| 静电纺丝法 | 适用于大多数MOF | 部分电纺丝系统需要在高腐蚀性或剧毒溶剂中进行,且有机溶剂成本高,不易回收,容易造成环境污染 |
| 非溶剂诱导相分离法以及热致相分离法 | 制备过程简单、快速,不需要复杂的设备和条件 | 仅适用于少数聚合物体系,且溶剂会造成环境污染 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
/
| 〈 |
|
〉 |