

Selective Hydrogenation of Acetylene: from Thermal Catalysis to Electrocatalysis, Photocatalysis and Photothermal Catalysis
Received date: 2023-11-03
Revised date: 2024-02-13
Online published: 2024-04-16
Supported by
National Natural Science Foundation of China(52372030)
National Natural Science Foundation of China(U23A20559)
National Natural Science Foundation of China(52272021)
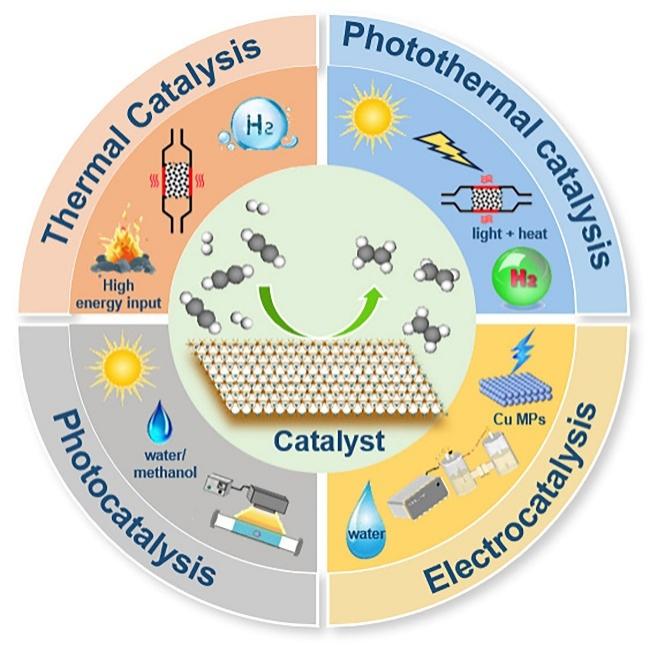
ethylene is one of the most important raw materials in the modern petrochemical industry.the preparation of ethylene by steam cracking of petroleum hydrocarbons generates acetylene with a volume fraction about 0.3%to 3%.These trace amounts of acetylene can poison the catalyst of the ethylene polymerization reaction.selective catalytic hydrogenation of acetylene is considered to be one of the most effective methods for removing acetylene impurities.This paper reviews the research progress of acetylene selective hydrogenation in recent years,introduces the reaction mechanism of acetylene hydrogenation,and summarizes the effects of catalyst active components,additives and carriers on the performance of acetylene selective hydrogenation.the development trend of how to further improve the performance of acetylene selective hydrogenation is discussed from the perspectives of electrocatalysis,photocatalysis and photothermal catalysis.Finally,some suggestions are proposed for the subsequent research on the selective hydrogenation of acetylene。
Contents
1 Introduction
2 Reaction mechanism of acetylene hydrogenation
3 Research progress of catalysts for thermocatalytic selective hydrogenation of acetylene
3.1 Catalyst active components and additives
3.2 Catalyst carriers
4 Trends in selective hydrogenation of acetylene
4.1 Electrocatalytic selective hydrogenation of acetylene and alkynes
4.2 Photocatalytic hydrogenation of acetylene and alkynes
4.3 Photothermal catalyzed hydrogenation of acetylene and alkynes
5 Conclusion and outlook
Baisheng Pang , Yingying Xing , Ruihong Gao , Yaohua Fang , Haijun Zhang , Liang Huang . Selective Hydrogenation of Acetylene: from Thermal Catalysis to Electrocatalysis, Photocatalysis and Photothermal Catalysis[J]. Progress in Chemistry, 2024 , 36(8) : 1237 -1253 . DOI: 10.7536/PC231104
图3 (A) 不同温度热处理下 PdCu 催化剂的 XRD 图谱,(B) PdCu-375 样品的 X 射线衍射 Rietveld 精修图谱,(C) 采用RIR法测定PdCu样品的面心立方/体心立方结构(FCC/BCC)比值,(D) PdCu/C、(E) PdCu/C-250和(F) PdCu/C-375的HRTEM图像[41]Fig.3 (A) XRD patterns of PdCu catalysts with different heat treatment temperatures. (B) Pattern of X-ray diffraction Rietveld refinement for the PdCu-375 samples. (C) FCC/BCC ratio of PdCu samples by the RIR method. HRTEM images of PdCu/C (D), PdCu/C-250 (E), and PdCu/C-375 (F)[41]. Copyright 2021, American Chemical Society |
表1 Performance Comparison of Different Catalysts in Thermal Catalytic Acetylene Selective HydrogenationTable 1 Performance comparison of different catalysts in thermally catalyzed selective hydrogenation of acetylene |
| Carrier | Active constituent | Assistant | Synthesis method | Reaction temperature/℃ | Acetylene conversion/% | Ethylene selectivity/% | ref |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | Pd | Impregnation | 105 | 80.9 | 76.9 | 26 | |
| ZnO | Pd | Zn | Impregnation | 80 | 92 | 89 | 27 |
| ZnO | Pd | Incipient wetness impregnation | 100 | 100 | >80 | 29 | |
| CNT | Pd | Cyclic voltammetry | 40 | 83 | 98 | 30 | |
| CeO2 | Pd | Co-precipitation and adsorption | 160 | 100 | 85 | 31 | |
| MCN | Pd | Ag | Template method | 200 | 99.8 | 98.1 | 40 |
| C | Pd | Cu | Impregnation | 100 | 100 | 86 | 41 |
| SiO2 | Ni | In | Impregnation | 180 | 100 | >60 | 47 |
| SiO2 | Ni | Ga | Impregnation | 180 | 100 | 75~81 | 48 |
| C | Ni | Zn | Impregnation pyrolysis | 160 | 100 | 85 | 55 |
| TiO2 | Pd | Hydrothermal and impregnation | 60 | 92 | 57 | 60 | |
| CNA | Au | Deposition-precipitation | 300 | 24 | 95 | 64 | |
| ZnO-Al2O3 | Pd | Ag | Co-precipitation and impregnation | 90 | 100 | 77.6 | 66 |
| TiO2/MCM-41 | Pd | Atomic layer deposition | 21 | 83.2 | 85.1 | 67 |
图8 (a)以甲醇悬浮液为氢源,苯乙炔在Pt/TiO2上光催化加氢为苯乙烯的示意图;(b)以H2作氢源的Pt/TiO2的比较实验;(c)苯乙炔在Pt/TiO2上选择性加氢反应的光催化反应机理[96]Fig. 8 (a) Schematic of photocatalytic hydrogenation of phenylacetylene to styrene on Pt/TiO2 using methanol suspension as a hydrogen source; (b) the comparative experiment over the Pt/TiO2 with H2 as hydrogenation source; (c) Proposed photocatalytic reaction mechanism for selective hydrogenation of phenylacetylene over Pt/TiO2[96]. Copyright 2020, Elsevier |
表2 Performance Comparison of Different Catalysts in Electrocatalytic, Photocatalytic and Photothermal Catalytic Selective HydrogenationTable 2 Comparison of the performance of different catalysts in electrocatalytic,photocatalytic and photothermal catalytic selective hydrogenation |
| Catalyst | Synthesis method | Hydrogen donors | Reaction condition | Catalytic performance | ref | |
|---|---|---|---|---|---|---|
| Electrocatalysis | Cu/C | Solvothermal | Water | T=25 ℃; V=4.8 mL·min-1 | $F E_{C_{2} H_{4}}$=83.2% | 68 |
| Cu/C | Electrochemical deposition | Water | T=25 ℃; $V_{\left(\mathrm{C}_{2} \mathrm{H}_{2}\right)} / V_{\left(\mathrm{C}_{2} \mathrm{H}_{4}\right)}$=1:99; SV=9.6×104 mL·gcat-1·h-1 | $F E_{C_{2} H_{4}}$=97% | 76 | |
| NHC-Cu | Impregnation | Water | T=25 ℃; $V_{\left(\mathrm{C}_{2} \mathrm{H}_{2}\right)} / V_{\left(\mathrm{C}_{2} \mathrm{H}_{4}\right)}$=1:99; SV=9.6×105 mL·gcat-1·h-1 | $F E_{C_{2} H_{4}}$=99%; TOF=2.1×10-2 s-1 | 80 | |
| SA-Ni-NC | Impregnation | Water | T=25℃; $V_{\left(\mathrm{C}_{2} \mathrm{H}_{2}\right)} / V_{\left(\mathrm{C}_{2} \mathrm{H}_{4}\right)}$=1:99; SV=2.4×104 mL·gcat-1·h-1 | $C_{C_{2} H_{2}}$=97.4%; $F E_{C_{2} H_{4}}$=91.3%; TOF=22.9 h-1 | 81 | |
| Cu SA/NC | Impregnation pyrolysis | Water | T=25 ℃; V=10 mL·min-1 | $F E_{C_{2} H_{4}}$=87.5% | 86 | |
| Zn-C2H2 | Electrochemical deposition | T=25 ℃; $V_{\left(\mathrm{C}_{2} \mathrm{H}_{2}\right)} / V_{\left(\mathrm{C}_{2} \mathrm{H}_{4}\right)}$=1:99 | $C_{C_{2} H_{2}}$=99.97%; $S_{c_{2} H_{4}}$=95% | 87 | ||
| Photocatalysis | Pt/TiO2 | Photodeposition method | Methanol | T=25 ℃; PLE=0.5 mmol | CPLE=92.4%; SSTE=91.3% | 96 |
| CoTPPS | Hydrogen | T=25 ℃; $V_{\left(\mathrm{C}_{2} \mathrm{H}_{2}\right)} / V_{\left(\mathrm{C}_{2} \mathrm{H}_{4}\right)}$=1:30 | $C_{C_{2} H_{2}}$=100%; $S_{c_{2} H_{4}}$=99% | 97 | ||
| Pd/TiO2 | Impregnation | Hydrogen | T=25 ℃; SV=3604 mL·gcat-1·h-1 | $C_{C_{4} H_{6}}$=99.0%; $S_{C_{4} H_{8}}$=98.0% | 98 | |
| Photothermal catalysis | Pd1/N-graphene | Freeze-drying | Hydrogen | T=125 ℃; $V_{\left(\mathrm{C}_{2} \mathrm{H}_{2}\right)} / V_{\left(\mathrm{H}_{2}\right)} / V_{\left(\mathrm{C}_{2} \mathrm{H}_{4}\right)}$=1:20:20; SV=1.2×103 mL·gcat-1·h-1 | $C_{C_{2} H_{2}}$=99%; $S_{c_{2} H_{4}}$=93.5% | 100 |
| Pd/TiO2 | Ball milling | Hydrogen | T=120 ℃; $V_{\left(\mathrm{C}_{2} \mathrm{H}_{2}\right)} / V_{\left(\mathrm{H}_{2}\right)} / V_{\left(\mathrm{C}_{2} \mathrm{H}_{4}\right)}$=1:10:20; SV=1.2×105 mL·gcat-1·h-1 | $C_{C_{2} H_{2}}$=99%; $S_{c_{2} H_{4}}$=64.5% | 101 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
/
| 〈 |
|
〉 |