

Catalytic Transformation of Biomass-Based Platform Compounds to Pentanediols
Received date: 2023-11-27
Revised date: 2024-02-14
Online published: 2024-04-16
Supported by
National Natural Science Foundation of China(22108038)
National Natural Science Foundation of China(21978248)
Foundation of State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology, Shandong Academy of Sciences(GZKF202308)
Science and Technology Plan Project of Quanzhou City(2022N030)
Natural Science Foundation of FujianProvince of China(2022J01573)
National Key R&D Program of China(2021YFC2101604)
Guangdong Provincial Key Research and Development Program(2020B0101070001)
pentanediols(1,2-pentanediol,1,4-pentanediol,and 1,5-pentanediol,etc.)are significant pharmaceutical chemicals that are used extensively worldwide.in addition to serving as an intermediate for the fungicide propiconazole and in the production of cosmetic products,they are also employed as key components in the manufacture of surfactants,polyester fibers,and pharmaceutical products.With the development of domestic and foreign markets in recent years,there has been growing demand for pentanediols production.Conventionally,the industrial production of pentanediols relies on non-renewable fossil resources,posing significant environmental concerns.Hence,the catalytic conversion of renewable biomass-based feedstocks to produce pentanediols represents a green production process,promising a brighter application prospect and research significance.This article provides a comprehensive review of recent studies on the catalytic transformation of biomass-based feedstocks(such as xylose,furfural,furfuryl alcohol,tetrahydrofurfuryl alcohol,gamma-valerolactone,and levulinic acid)for pentanediols production.A detailed summary of the catalysts employed for pentanediols synthesis is presented,encompassing various catalytic systems(noble and non-noble metals),reaction mechanisms,pathways,reaction conditions,and catalyst stability.on This basis,perspectives are offered on refining the biomass-derived pentanediols production process,considering sustainability and economics,etc.This review aims to furnish theoretical guidance and beneficial references for further developing novel,efficient,green,and stable catalytic systems。
1 Introduction
2 Preparation of pentanediol by catalytic conversion with different substrates
2.1 Preparation of 1,2-pentanediol by catalytic conversion of xylose
2.2 Preparation of pentanediol by catalytic conversion of furfural
2.3 Preparation of pentanediol by catalytic conversion of furfuryl alcohol
2.4 Preparation of pentanediol by catalytic conversion of tetrahydrofurfuryl alcohol
2.5 Preparation of 1,4-pentanediol by catalytic conversion of levulinic acid
2.6 Preparation of 1,4-pentanediol by catalytic conversion of gamma-valerolactone
3 Prospects for biomass-based production routes for the preparation of pentanediols
4 Conclusion and outlook
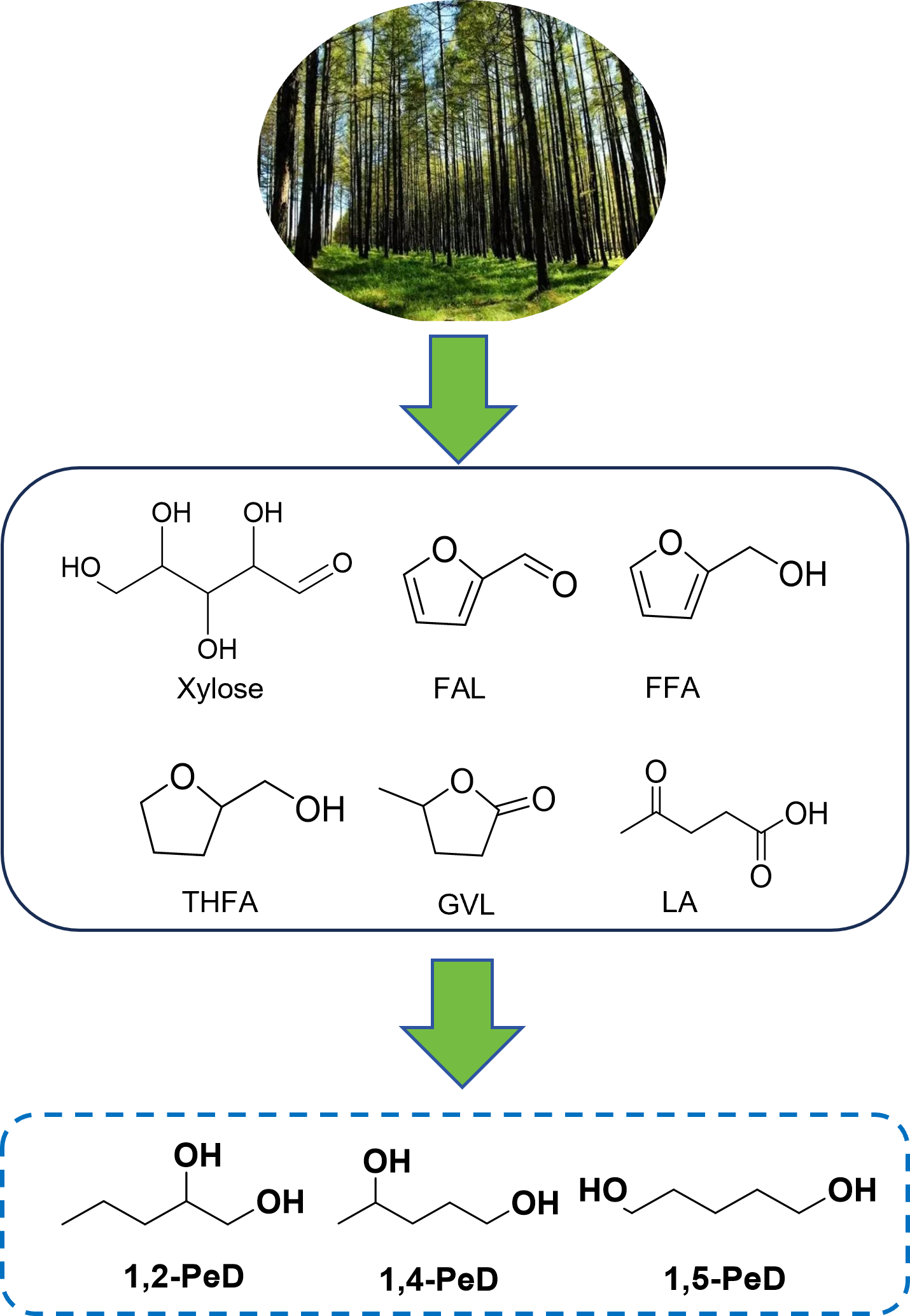
Key words: furfural; furfuryl alcohol; tetrahydrofurfuryl alcohol; levulinic acid; catalyst; pentanediols
Jiahui Deng , Yi Liu , Liangqing Zhang , Jiarong Qiu , Jianfeng Chen , Xianhai Zeng . Catalytic Transformation of Biomass-Based Platform Compounds to Pentanediols[J]. Progress in Chemistry, 2024 , 36(7) : 1061 -1087 . DOI: 10.7536/PC231118
表1 Preparation of 1,2-PeD and 1,5-PeD from FAL over Noble Metal CatalystsTable 1 Preparation of 1,2-PeD and 1,5-PeD from FAL by noble metal catalysts |
| Entry | Catalyst | Solvent | Reaction conditions | Conversion (%) | Selectivity (%) | Run (Stability) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| 1,2-PeD | 1,5-PeD | ||||||||
| 1 | Pt-Li /Co2AlO4 | Alcohol | 413 K, 1.5 MPa, 24 h | 99.9 | 16.2 | 34.9 | 3 (Stable) | 31 | |
| 2 | Pt/CeO2 | Water | 443 K, 1 MPa, 1.5 h | 100 | 65.0 | 8 | / | 30 | |
| 3 | Pt/CeO2 | Isopropanol | 438 K, 1.5 MPa, 4 h | 100 | 59.9 | 3.1 | 5 (Stable) | 32 | |
| 4 | Pt/HT | Isopropanol | 423 K, 3 MPa, 4 h | 100 | 73 | 8 | 3 (Stable) | 33 | |
| 5 | Pt/MgO | Isopropanol | 423K, 3 MPa, 4 h | 100 | 68 | 7 | / | 33 | |
| 6 | Pt/γ-Al2O3 | Isopropanol | 423 K, 3 MPa, 4 h | 100 | 28 | 4 | / | 33 | |
| 7 | Pt/ReOx/TiO2 | Isopropanol | 403 K, 6 MPa, 8 h | 100 | 7.28 | 29.72 | / | 34 | |
| 8 | Pd-Ir-ReOx /SiO2 | Water | 313/373 K, 8 MPa, 8 h/72 h | >99.9 | 1.4 | 71.4 | 4 (Stable) | 35 | |
| 9 | Rh-Ir-ReOx /SiO2 | Water | 313/373 K, 8 MPa, 8 h/32 h | >99.9 | 1.2 | 78.2 | 3 (Unstable) | 38 | |
| 10 | Rh/OMS-2 | Methanol | 433 K, 3 MPa, 8 h | 99.6 | 87 | / | 4 (Stable) | 39 | |
| 11 | 2Pd-2Au/Ti-SBA-15 | Isopropanol | 433 K, 2 MPa, 5 h | 93 | 59 | / | 3 (Stable) | 36 | |
| 12 | 2Pd-2Au/Ti-HMS | Isopropanol | 433 K, 2 MPa, 5 h | 88 | 51 | / | 3 (Stable) | 36 | |
| 13 | Pd/(MMT-K 10) | Isopropanol | 493 K, 3.5 MPa, 5 h | >99.9 | 66 | / | 2 (Stable) | 37 | |
表2 Preparation of 1,2-PeD and 1,5-PeD from FFA catalyzed by noble metal catalystTable 2 Preparation of 1,2-PeD and 1,5-PeD from FFA by noble metal catalysts |
| Entry | Catalyst | Solvent | Reaction conditions | Conversion (%) | Selectivity (%) | Run (Stability) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|
| 1,2-PeD | 1,5-PeD | ||||||||
| 1 | Pt /HT | Isopropanol | 423 K, 3 MPa, 4 h | 100 | 80.0 | / | / | 33 | |
| 2 | Pt/CeO2 | Ethanol | 438 K, 2 MPa, 24h | >99.9 | 77.1 | 7.3 | 5 (Stable) | 45 | |
| 3 | Pt / Al2O3 | Water | 443 K, 1 MPa, 1.5 h | 100 | 19.2 | 5.9 | / | 30 | |
| 4 | Pt / La-Al2O3 | Water | 443 K, 1 MPa, 1.5 h | 100 | 41.6 | 4.5 | / | 30 | |
| 5 | Ru /MnOx | Water | 423 K, 1.5 MPa, 4 h | 89.2 | 41.4 | / | 4 (Stable) | 2 | |
| 6 | Ru /Al2O3 | Water | 473 K, 10 MPa, 1 h | 100 | 32.0 | / | / | 46 | |
| 7 | Ru-Mn/CNTs | Water | 423 K, 4 MPa, 4 h | 86.2 | 20.1 | / | 5 (Stable) | 47 | |
| 8 | Ru/MgO | Water | 463 K, 3 MPa, 1 h | 100 | 42.0 | 2.9 | / | 48 | |
| 9 | Ru/Mn0.67Al0.33 | Water | 423 K, 2 MPa, 10 h | 99.6 | 48.2 | / | 11 (Stable) | 49 | |
| 10 | Ru/Mn0.67Al0.33 | / | 423 K, 2 MPa, 22 h | 98.5 | 42.3 | 7 (Stable) | 49 | ||
表3 Preparation of 1,2-PeD and 1,5-PeD from FFA catalyzed by non-noble metal catalystTable 3 Preparation of 1,2-PeD and 1,5-PeD from FFA by non-noble metal catalysts |
| Entry | Catalyst | Solvent | Reaction conditions | Conversion (%) | Selectivity (%) | Run (Stability) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|
| 1,2-PeD | 1,5-PeD | ||||||||
| 1 | CuCr2O4 | / | 448 K, 10~15 MPa, 11.5 h | / | 40.0a) | 30.0a) | / | 40 | |
| 2 | Cu-Mg3AlO4.5 | Ethanol | 413 K, 6 MPa, 24 h | >99 | 51.2 | 28.8 | 6 (Stable) | 50 | |
| 3 | Cu-Al2O3 | Ethanol | 413 K, 6 MPa, 8 h | 60.4 | 48.6 | 22.7 | 5 (Stable) | 51 | |
| 4 | 0.1Cu-2.9Co-Al | Ethanol | 433 K, 4 MPa, 2 h | 97.8 | 18.9 | 44.7 | / | 42 | |
| 5 | Cu-LaCoO3 | Ethanol | 413 K, 6 MPa, 2 h | 100 | 15.2 | 40.3 | 4 (Stable) | 56 | |
| 6 | 60CuO40ZrO2 | Water | 443 K, 9 MPa, 6 h | 28.0 | 34.0 | 10.0 | / | 52 | |
| 7 | Cu@MgO-La2O3 | Isopropanol | 413 K, 6 MPa, 24 h | 94.9 | 67.1 | 18.8 | 6 (Stable) | 53 | |
| 8 | Co-TiO2 b) | Water | 413 K, 2.34 MPa, WHSV = 5.8 h−1 | 99.0 | 2.4 | 30.3 | 3 (Stable) | 54 | |
| 9 | Ni-Y2O3 | Isopropanol | 423 K, 3 MPa, 24 h | 100 | <2 | 47.8 | 2 (Unstable) | 55 | |
a)Yield;b)Fixed-bed reactor,WHSV=5.8 h−1 |
表4 Preparation of 1,2-PeD and 1,5-PeD from THFA over Noble Metal CatalystsTable 4 Preparation of 1,2-PeD and 1,5-PeD from THFA by noble metal catalysts |
| Entry | Catalyst | Solvent | Reaction conditions | Conversion (%) | Selectivity (%) | Catalyst stability | Ref | |
|---|---|---|---|---|---|---|---|---|
| 1,2-PeD | 5-PeD | |||||||
| 1 | Rh-ReOx/SiO2 | Water | 393 K, 8 MPa, 24 h | 96.0 | 0.0 | 80.0 | 5 (Unstable)a) | 58 |
| 2 | Rh-MoOx/SiO2 | Water | 373 K, 8 MPa, 24 h | 94.2 | 0.0 | 90.3 | 5 (Stable)a) | 61 |
| 3 | Rh-ReOx/C | Water | 373 K, 8 MPa, 24 h | 99.0 | 0.0 | 94.0 | / | 59 |
| 4 | Rh-ReOx/C | Water | 393 K, 3.4 MPa, 4 h | 47.2 | 0.0 | 97.2 | 120 hb) | 64 |
| 5 | Rh-MoOx/C | Water | 393 K, 3.4 MPa, 4 h | 51.6 | 0.0 | 91.3 | / | 64 |
| 6 | Rh/C | Water | 393 K, 3.4 MPa, 4 h | 8.5 | 20.7 | 59.1 | / | 64 |
| 7 | Rh/SiO2+MoO3 | Water | 393 K, 6 MPa,20 h | 27.9 | - | 80.3 | 4 (Stable) | 62 |
| 8 | Rh-MCM-41 | SCO2 | 353K, 4 MPa, 24 h | 80.5 | 0.0 | 91.2 | / | 57 |
| 9 | 5%Rh-4%Mo/ZrO2 | Water | 393K, 8 MPa, 24 h | 90.0 | - | 90 | 2 (Unstable) | 65 |
| 10 | Ir-ReOx/SiO2 | Water | 373 K, 8 MPa, 8 h | 94.0 | 0.0 | 87.0 | / | 60 |
| 11 | Ir-MoOx/SiO2 | Water | 393 K, 6 MPa, 6 h | 75.0 | 0.0 | 65.0 | 30 h | 66 |
| 12 | Ir-VOx /SiO2 | Water | 353 K, 6 MPa, 6 h | 57.0 | 0.0 | 89.0 | / | 67 |
| 13 | Pt /WO3@SiO2 | Water | 493 K, 6 MPa, 24 h | 82.9 | 0.0 | 72.9 | 4 (Stable)c) | 68 |
| 14 | Pt/WO3/ZrO2 | Isopropanol | 423 K, 5 MPa, 5 h | 56.0 | 0.0 | 65.0 | / | 69 |
| 15 | Pt/Y2O3-WO3-ZrO2 | Water | 423K, 4 MPa, WHSV=0.2 h−1 | 88.0 | 0.0 | 77.3 | 100 h | 71 |
a)Reaction conditions:393 K,8.0 MPa H2,and 12 h;b)Reaction conditions:Reduced under H2at 523 K for 4 h;c)Reaction conditions:473 K,6.0 MPa H2,and 12 h |
表5 Preparation of 1,2-PeD and 1,5-PeD from THFA catalyzed by non-noble metal catalystTable 5 Preparation of 1,2-PeD and 1,5-PeD from THFA by non-noble metal catalysts |
| Entry | Catalyst | Solvent | Reaction conditions | Conversion (%) | Selectivity (%) | Run (Stability) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|
| 1,2-PeD | 5-PeD | ||||||||
| 1 | Cu-Cr | Water | 453 K, 8 MPa, 4 h | 0.6 | 9.4 | 0.0a | / | 58 | |
| 2 | RaneyNi | Water | 453 K, 8 MPa, 4 h | 0.2 | 12.7 | 13.1 | / | 58 | |
| 3 | Ni /Al2O3a) | Water | 523 K, 5.5 MPa | 17.3 | 0.0 | 59.2 | / | 23 | |
| 4 | Ni-Y2O3 | Isopropanol | 423 K, 2 MPa, 24 h | 59.8 | - | 54.7b) | / | 55 | |
| 5 | Ru-Ni-Y2O3 | Isopropanol | 423 K, 2 MPa, 40 h | 93.4 | - | 86.5b) | / | 72 | |
| 6 | Ni-WOx/SiO2 | Water | 523 K, 3.4 MPa, 4 h | 28.7 | 0.0 | 47.3 | 3 (Unstable) | 73 | |
a)High-throughput reactor(HTR),TOF=0.004 s−1;b)Yield |
表6 Preparation of 1,4-PeD from LA catalyzed by noble metal catalystTable 6 Preparation of 1,4-PeD from LA by noble metal catalysts |
| Entry | Catalyst | Solvent | Reaction conditions | Conversion (%) | yield (%) | Catalyst stability | Ref |
|---|---|---|---|---|---|---|---|
| 1 | (Triphos)Ru(CO)(H)2 | / | 433 K, 10 MPa, 18 h | / | 73 | / | 78 |
| 2 | Ru(acac)3+Triphos | / | 433 K, 10 MPa, 18 h | 100 | 95 | / | 78 |
| 3 | Ru-N-Triphos | Tetrahydrofuran | 423 K, 6.5 MPa, 25 h | 100 | 99 | / | 79 |
| 4 | Ru-Re/C | Water | 413 K, 15 MPa, 28 h | 100 | 82 | / | 80 |
| 5 | Ru-MoOx /AC | Water | 343 K, 4 MPa, WHSV=0.4 h−1 | 99.9 | 96.7 | 200 h | 74 |
| 6 | Rh-MoOx/SiO2 | Water | 353 K, 6 MPa, 12 h | 100 | 70 | 30 h | 82 |
| 7 | Ir-MoOx/SiO2 | Water | 373 K, 6 MPa | 100 | 42.3 | / | 83 |
| 8 | Pt-Mo/HAP | Water | 403 K, 5 MPa, 12 h | >99 | 93 | 2 (Stable) | 75 |
表7 Preparation of 1,4-PeD from LA/EL over non-noble metal catalystTable 7 Preparation of 1,4-PeD from LA/EL by non-noble metal catalysts |
| Entry | Catalyst | Solvent | Reaction conditions | Conversion (%) | yield (%) | Run (Stability) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Cu/Cr2O3 | / | 573 K, 20 MPa, 1.3 h | / | 44 | / | 84 |
| 2 | FeCuB | 1,4-Dioxane | 473 K, 5 MPa, 6 h | 100 | 85.1 | / | 85 |
| 3 | CuZnAl | 1,4-Dioxane | 433 K, 6 MPa, 6 h | 100 | 98 | / | 86 |
| 4 | CuCo/Al2O3 | 1,4-Dioxane | 413 K, 24 h | / | 88 | / | 76 |
| 5 | Cu-Ni-Zn/H-ZSM-5 | Water | 403 K, 2.5 MPa, 6 h | 97.4 | 93.4 | 5 (Stable) | 87 |
| 6 | Co/ZrO2 | 1,4-Dioxane | 463 K, 8 MPa, 8 h | 100 | 78 | 12 (Stable) | 89 |
| 7 | Co-Mg-Al | Isopropanol | 423 K, 4 MPa, 10 h | 100 | 98 | 4 (Stable) | 88 |
表8 Preparation of 1,4-PeD by GVL catalyzed by non-noble metal catalystTable 8 Preparation of 1,4-PeD from GVL by non-noble metal catalysts |
| Entry | Catalyst | Solvent | Reaction conditions | Conversion (%) | Selectivity (%) | Run (Stability) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | 30-Cu/ZrO2-OG | Ethanol | 473 K, 6 MPa, 6 h | 97 | 99 | 3 (Stable) | 92 |
| 2 | Cu-TiO2/ZrO2-CP-600 | Ethanol | 473 K, 5 MPa, 6 h | 27 | 96 | / | 93 |
| 3 | 40-Cu/ZnO-CP | / | 413 K, 1.5 MPa, 10 h | 82.3 | 99.2 | / | 91 |
| 4 | 18%Cu/MgO | 1,4-Dioxane | 473 K, 10 MPa, 10 h | 90.5 | 94.4 | 3 (Stable) | 94 |
| 5 | Cu1.5/Mg1.5AlO | 1,4-Dioxane | 433 K, 5 MPa, 12 h | 93 | >99 | 5 (Stable) | 95 |
| 6 | Cu/LaCoO3 | / | 513 K, 7 MPa, 10 h | 92.5 | 92.1 | / | 96 |
| 7 | Cu/SiO2 | Butyl alcohol | 403 K, 1.3 MPa, 9 h | 32 | 67 | / | 97 |
| 8 | Cu/Al2O3 | 1,4-Dioxane | 473 K, 4 MPa, 3 h | 98 | <20 | 5 (Unstable) | 12 |
| 9 | Zn1.5Cu/Al2O3 | 1,4-Dioxane | 473 K, 4 MPa, 2 h | 91 | 97 | 10 (Stable) | 12 |
| 10 | Cu0.3Zn/Al2O3 | 1,4-Dioxane | 473 K, 4 MPa, 2 h | 62 | 98 | / | 12 |
| 11 | Ni-MoOx/Al2O3-600 | Mesitylene | 433 K, 4 Mpa, 4 h | 82 | 78 | / | 99 |
| 12 | Ni-MoOx-500 | Water | 433 K, 4 MPa, 4 h | 70 | 92 | / | 99 |
| 13 | (PNNH)CoCl2 | Tetrahydrofuran | 403 K, 5 MPa, 70 h | / | 50a) | / | 100 |
| 14 | Co/ZrO2-CP-750 | Water | 438 K, 5 MPa, 24 h | 86.1 | 97.2 | 3 (Stable) | 101 |
a)yield(% ) |
| [1] |
(高芳芳, 陈静, 黄志威, 夏春谷. 分子催化, 2018, 32: 276.).
|
| [2] |
|
| [3] |
|
| [4] |
(樊冬娜, 刘晓然, 王喜成, 于奕峰, 陈爱兵. 化工进展, 2018, 37: 938.).
|
| [5] |
|
| [6] |
(王庄清, 张弨, 赵凤玉. 科学通报, 2019, 64(31): 3165.).
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
(梁缘. 中国科学技术大学硕士论文, 2021.).
|
| [11] |
|
| [12] |
(刘强. 长春工业大学硕士论文, 2020.).
|
| [13] |
(罗岩. 湖北工业大学硕士论文, 2011.).
|
| [14] |
(匡碧锋. 广东工业大学硕士论文, 2020.).
|
| [15] |
|
| [16] |
(张正凯, 邓一健, 栾振中. 精细与专用化学品, 2004, 12(22): 18.).
|
| [17] |
|
| [18] |
(罗岩, 徐保明, 汤国亮, 陈坤. 农药, 2010, 49: 161.).
|
| [19] |
(许彦娟. 浙江大学硕士论文, 2014.).
|
| [20] |
(席荣. 宁夏大学硕士论文, 2021.).
|
| [21] |
(梁缘, 李志坚, 刘琪英, 马隆龙. 燃料化学学报, 2021, 49: 1898.).
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
(谭静静, 苏以豪, 高宽, 崔静磊, 王永钊, 赵永祥. 燃料化学学报, 2021, 49: 780.).
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
(白小薇, 王梦菲, 邱腾龙, 郭丽, 李建. 新疆大学学报(自然科学版), 2023, 40: 313.).
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
(王新承, 杨靖丰, 郑枝源, 黄龙, 易玉峰, 高乐, 杜松松. 工业催化, 2022, 30: 60.).
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
(丁璟, 赵俊琦, 程时标, 慕旭宏, 宗保宁. 化工进展, 2015, 34: 4209.).
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
(杨晓, 陈长林. 南京工业大学学报(自然科学版), 2019, 41: 149.).
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
(吕金昆. 大连理工大学博士论文, 2018.)
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
(宋会. 化工管理, 2018, 72.)
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
(刘强, 赵振波, 张弨, 赵凤玉. 应用化学, 2020, 37: 1285.).
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
/
| 〈 |
|
〉 |