

Crystal Material Construction Based on DNA Nanotechnology
Received date: 2024-01-17
Revised date: 2024-02-29
Online published: 2024-07-01
Supported by
National Natural Science Foundation of Jiangsu Province, China(BK20220124)
National Natural Science Foundation of China(22372077)
National Natural Science Foundation of China(92356304)
State Key Laboratory of Analytical Chemistry for Life Science,Nanjing University(5431ZZXM2301)
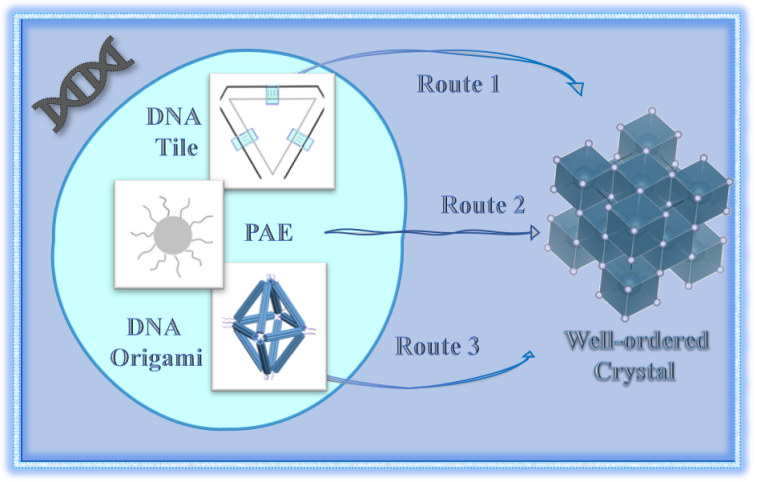
nanocrystal materials have exhibited unique and superior properties in the fields of electronics,optics,and magnetism.the fabrication of nanocrystal materials holds significant importance in the material science for achieving breakthroughs and further developments in their performance.DNA,known for its complementary base pairing characteristics,offers the potential to construct diverse nanostructures and crystals of which the structures and composition can be precisely controlled,thereby enabling the tailored material properties.Currently,the nanocrystal materials fabricated through DNA nanotechnology has been applied in various fields,including catalysts,optical devices,and semiconductor materials.These advancements signify a progressive realization of the fundamental objective for constructing three-dimensional crystals as versatile,periodic molecular frameworks.in this review,we provide a systematic elucidation of the development and recent advancements in the research pertaining to three pivotal techniques employed in the construction of DNA nanocrystals.These techniques include DNA tiles,programmable atomic equivalents,and DNA origami.Moreover,we briefly deliberate on the future direction of utilizing DNA nanotechnology in the construction of nanocrystal materials。
1 Introduction
2 Construction of nanoparticle crystals based on DNA tiles
3 Direct DNA-mediated nanoparticle crystallization
4 DNA origami-based assembly technique for nanoparticle crystal
5 Conclusion and outlook
Peixin Li , Mushi Peng , Xuehui Yan , Yifan Yu , Ye Tian . Crystal Material Construction Based on DNA Nanotechnology[J]. Progress in Chemistry, 2024 , 36(7) : 949 -960 . DOI: 10.7536/PC240116
图2 (a)阿基米德图形及构成其的DNA瓦片[19];(b)三种链构成的“六点星”DNA瓦片及其组装的六边形晶格示意图[6];(c)张拉整体三角形示意图[25];(d)使用DNA拉张整体三角形构建的菱方(R3)和六方晶格(P63)[10]Fig. 2 (a) Archimedean shapes and the DNA tiles composing them[19], Copyright © 2013, American Chemical Society; (b) A schematic diagram of a hexagonal lattice composed of “six-pointed star” DNA tiles consisting of three strands[6], Copyright © 2006, American Chemical Society; (c) A schematic diagram of DNA tensegrity triangles[25]; (d) Diamond (R3) and hexagonal (P63) lattices constructed using DNA tensegrity triangles[10], Copyright © 2021, American Chemical Society. |
图3 (a)PAE组成及构成晶体示意图[31];(b)通过改变DNA连接体的序列将金纳米粒子-DNA偶联物编程组装成不同的晶体排列[60];(c)pH变化引发的晶格收缩和膨胀[40];(d)EEs与PAE组成的四种胶体晶体合金等价物[61]Fig. 3 (a) Schematic diagram of PAE composition and crystal structure[31], Copyright © 2022 Wiley‐VCH GmbH; (b) Programmed assembly of gold nanoparticles DNA conjugates into different crystal arrangements by changing the sequence of DNA linkers[60], Copyright © 2013 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim; (c) Lattice contraction and expansion caused by pH changes[40], Copyright © 2018, American Chemical Society; (d) Four colloidal crystal alloy equivalents composed of EEs and PAE[61], Copyright © 2019, American Chemical Society |
图4 (a)DNA折纸术的发明[95];(b)多层DNA折纸组成蜂窝状晶格[96];(c)不同DNA折纸框架自组装形成晶格[86];(d)利用DNA折纸八面体制备Wulff形状的单晶[94],比例尺:1 μmFig. 4 (a) The invention of DNA origami[95]; (b) Multiple layers of DNA origami form a honeycomb lattice[96],Copyright © 2020, Springer Nature Switzerland AG; (c) Different DNA origami frameworks self assemble to form a lattice[86], Copyright © 2016, Springer Nature Limited; (d) Preparation of Wulff shaped single crystals using DNA origami octahedra[94],scale bar:1 μm |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
/
| 〈 |
|
〉 |