

Intracellular Single Strand DNA and High-Throughput Analysis Techniques
Received date: 2024-03-04
Revised date: 2024-03-30
Online published: 2024-04-16
Supported by
National Natural Science Foundation of China(22234008)
National Natural Science Foundation of China(21927807)
National Natural Science Foundation of China(22021003)
National Natural Science Foundation of China(22274166)
During many life processes such as replication,transcription,double-strand breaks repair and so on,double-stranded DNA will temporarily unwind and form single strand DNA(ssDNA).ssDNA may affect genomic stability and may also participate in the formation of non-B DNA structure,which in turn regulates and influences many key cellular processes.This review briefly describes the causes of the formation of single-stranded DNA,the structures containing single-stranded DNA and their possible functions in cells,and summarizes some high-throughput analysis techniques of single-stranded DNA,which provides the method inspiration for the subsequent ssDNA research and promotes the further development of ssDNA analysis techniques and methods。
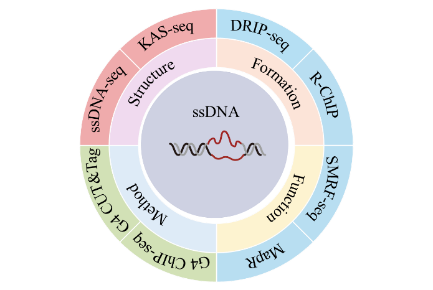
1 Overview of ssDNA
2 Formation and function of ssDNA
3 ssDNA sequencing methods
3.1 ssDNA-seq
3.2 KAS-seq
3.3 DRIP-seq
3.4 R-ChIP
3.5 SMRF-seq
3.6 MapR
3.7 G4 ChIP-seq
3.8 G4 CUT&Tag
4 Conclusion and outlook
Key words: single strand DNA; R-loop; G-quadruplex; high-throughput sequencing
Ruiqi Li , Weiyi Lai , Hailin Wang . Intracellular Single Strand DNA and High-Throughput Analysis Techniques[J]. Progress in Chemistry, 2024 , 36(9) : 1283 -1290 . DOI: 10.7536/PC240304
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
/
| 〈 |
|
〉 |