

Cu-Based Catalysts for Electrocatalytic Nitrate Reduction
Received date: 2024-01-26
Revised date: 2024-03-04
Online published: 2024-04-16
Supported by
New Energy Material Innovation Consortium Projects of Yunnan Province(202302AB080018)
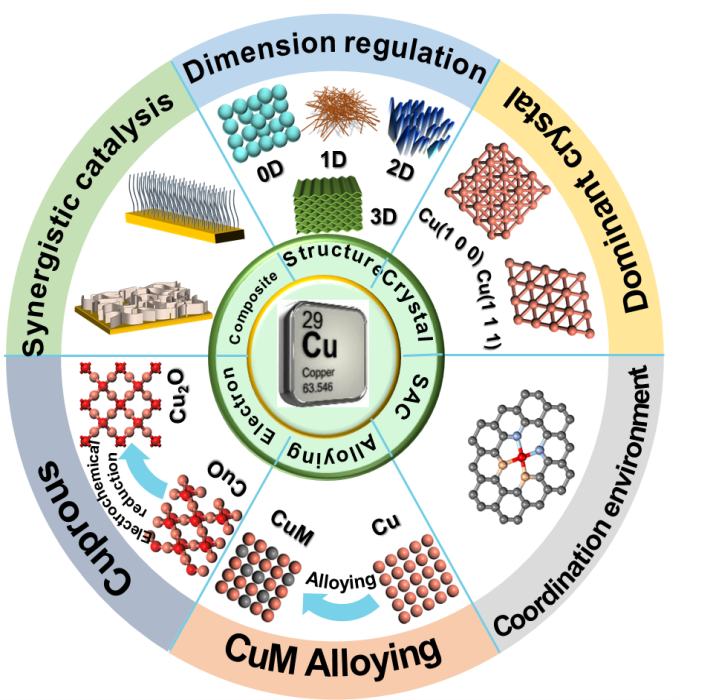
in recent years,electrocatalytic nitrate reduction(ENitRR)has attracted considerable attention in the synthesis of ammonia at ambient conditions.Compared to the traditional Haber-Bosch process for ammonia synthesis,ENitRR offers lower energy consumption and milder reaction conditions.the design and optimization of ENitRR electrocatalysts are crucial for nitrate deoxygenation and hydrogenation.copper-based catalytic materials have been widely studied due to their unique structure,low cost,and excellent performance,making them highly promising electrocatalysts through various morphology and electronic structure modulation strategies.This article summarizes various effective design strategies using copper-based electrocatalysts as a typical example to enhance the ammonia production rate and conversion efficiency in ENitRR.It also introduces the reaction mechanism and the relationship between structural changes in Cu-based electrocatalysts and their performance.These strategies include morphology modulation,alloy engineering,lattice phase tuning,single-atom structures,as well as copper compound construction and composites with other materials.Finally,challenges faced by copper-based electrocatalysts are discussed along with future research directions that should be focused on in order to provide reference for researchers engaged in nitrate treatment in aqueous systems。
1 Introduction
2 Mechanism of ENitRR
3 Research status of Cu-based electrocatalysts
3.1 Metal Copper(Cu0 )
3.2 Cuprous based catalyst
3.3 Copper matrix composite
4 Conclusion and outlook
Changzheng Lin , Jinwei Zhu , Weijia Li , Hao Chen , Jiangtao Feng , Wei Yan . Cu-Based Catalysts for Electrocatalytic Nitrate Reduction[J]. Progress in Chemistry, 2024 , 36(9) : 1291 -1303 . DOI: 10.7536/PC240123
图2 析氢反应、氮还原反应(NRR)和ENitRR的电化学还原电极电势值Fig. 2 Electrochemical reduction electrode potential values for hydrogen evolution reaction, nitrogen reduction reaction (NRR) and ENitRR |
图3 通过对比表面积、晶面、配位环境、合金化、价态调控和与复合材料提升铜基电催化剂的ENitRR性能Fig. 3 The electrocatalytic performance of Cu-based catalysts was improved by optimizing factors such as surface area, crystal plane orientation, coordination environment, alloying strategies, valence regulation, and composite materials |
图6 (a)铜纳米颗粒(0D)[75]、(b)铜纳米片(1D)[80]、(c)铜纳米线(2D)[77]和(d)多孔铜结构(3D)[78]的SEM图Fig. 6 SEM images of (a) copper nanoparticles (0D) [75], (b) copper nanosheets (1D) [80], (c) copper nanowires (2D) [77], and (d) porous copper structures (3D) [78]. Copyright ©, John Wiley & Sons, Ltd; Copyright ©, American Chemical Society |
表1 Performance Summary of Copper Nanostructures with Different Dimensions for Electrochemical Nitrate Reduction to AmmoniaTable 1 A comprehensive review on electrochemical nitrate reduction for ammonia synthesis using copper nanostructures of varying dimensions |
| Dimension | Cathode | Performance | ref |
|---|---|---|---|
| 0D | Copper nanoparticles | 73%~74% NO3− conversion rate; 97% NH3 selectivity | 79 |
| 1D | Copper nanowire | NO2− and NH3 with current efficiencies of 91.5% and 100% | 77 |
| 2D | Copper nanosheet | 390.1 μg·mg−1 Cu h−1 ammonia rate; 99.7% Faradaic efficiency | 76 |
| 3D | 3D foam Cu-catalyst | Partial current densities beyond 1 A·cm-2 | 78 |
图8 (a)NO3−和NO2−在Cu(111)、Cu-N4和Cu-N2表面上的自由能[46];(b)Cu 单原子应用电位驱动的聚集机制和氧化环境中的再分散[86]Fig. 8 (a) Free energies of NO3− and NO2− on Cu(111), Cu-N4 and Cu-N2 surfaces; (b) Potential driven aggregation mechanisms of Cu SAC application and redispersion in an oxidative environment[86]. Copyright ©2022, John Wiley & Sons, Ltd, Copyright ©2022, American Chemical Society |
表2 Summary of Ammonia Synthesis by Electrochemical Nitrate Reduction with Different Metals and Copper Alloying StructuresTable 2 Summary of electrochemical nitrate reduction for ammonia synthesis using various metal and copper alloy structures |
| metal | Cathode | Performance | ref |
|---|---|---|---|
| Precious metals | Ru@Cu | 162 mA·cm−2 for NH3 production;Faradaic efficiency of 93% | 88 |
| Ru-Cu NW | Faradaic efficiency of 93%; Industrial-relevant nitrate reduction current of 1 A·cm-2 | 39 | |
| PA-RhCu cNCs | Faradaic efficiency of 93.7%; NH3 production yield of 2.40 mg·h−1 mg·cat−1 | 45 | |
| RuxCuy/ rGO | Faradic efficiency of 98%; Ammonia formation rate of 0.38 mmol·cm−2·h−1 | 89 | |
| PdCu MSs | Faradaic efficiency of 85%; yield rate of 3058 µg·h−1·mg−1 | 90 | |
| Cu-Pt | yielding 194.4 mg NH3-N L−1·gcat−1, SNH3 of 84% | 91 | |
| Non-precious metals | Cu50Ni50 | 0.12 V upshift in the half-wave potential; 6-fold increase in activity | 35 |
| Ni1Cu- SAA | Faradaic efficiency of ~100%; NH3 yield rate of 326.7 μmol·h−1·cm−2 | 50 | |
| CuCo nanosheet | Faradaic efficiency of 100 %± 1%; 4.8 mmol·cm−2·h−1 of NH3 production rate | 64 | |
| Cu49Fe1 | Faradaic efficiency up to 94.5 %; Selectivity of 86.8 %. | 92 | |
| Fe/Cu | Faradaic efficiency of 92.51%; NH3 yield rate of 1.08 mmol·h−1·mg−1 | 93 | |
| Zn/Cu | Faradaic efficiency of 98.4%; NH3 yield rate of 5.8 mol·g−1·h−1 | 94 |
图10 (a)Cu@Cu2+1O NWs的电催化硝酸盐还原机制示意图;(b)氢气吸附模型和生成氢气的反应能;(c)反应自由能[99]Fig. 10 (a) Schematic illustration showing the electrocatalytic nitrate reduction mechanism over the Cu@Cu2+1O NWs. (b) Hydrogen adsorption model and reaction energy of hydrogen generation. (c) free energy of reaction[99]. Copyright@2022, American Chemical Society |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
2023 Research Frontier, Institute of Science and Technology Strategy Consulting, Chinese Academy of Sciences, National Science Library, Chinese Academy of Sciences, Clarivate Analytics, 2023.
(2023研究前沿, 中国科学院科技战略咨询研究院, 中国科学院文献情报中心, 科睿唯安, 2023.)
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
/
| 〈 |
|
〉 |