

Ternary Random Strategy Applied in Active Layer of High Performance Non-Fullerene Organic Solar Cells
Received date: 2024-02-05
Revised date: 2024-05-26
Online published: 2024-07-01
Supported by
National Natural Science Foundation of China(51573026)
National Natural Science Foundation of China(22261002)
National Natural Science Foundation of China(61804065)
Natural Science Foundation of Fujian Province(2020J01144)
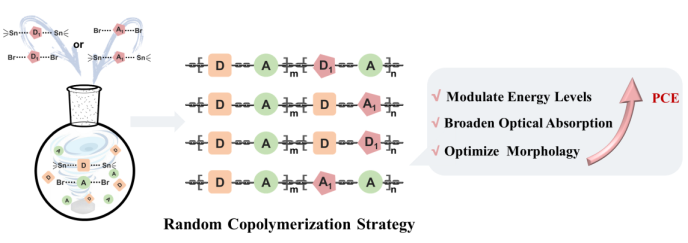
in recent years,there has been significant progress in non-fullerene organic solar cells(NF-OSCs)due to the rapid development of narrow-bandgap small-molecule acceptor materials and the high-performance polymer donor materials,with the power conversion efficiency(PCE)approaching 20%.However,As the design of alternating D-A copolymer materials reaches saturation,there is an urgent need to develop more efficient conjugated polymer materials.the ternary random strategy has emerged to address this challenge.the advantages of the ternary random copolymerization,including easy energy level tuning,broad and strong absorption,and high molar absorptivity,which have attracted considerable attention in the field of organic solar cells.In this review,firstly,the advantages of the ternary random copolymerization strategy in modulating polymer properties and device performance are discussed.Through this strategy,the active layer morphology can be effectively regulated and optimized,and thus the charge transfer efficiency can be improved leading to the improved PCE.Furthermore,the application of the ternary random copolymerization into NF-OSCs is summarized from the perspectives of random polymer donors and acceptors.Finally,a summary and outlook of the further development of random polymers are presented.as expected,to understand the design concept and advantages of ternary random strategy would be beneficial for the development of organic solar cells。
1 Introduction
2 Advantages of random copolymerization strategy
3 Random polymer donors
3.1 PBDB series random polymer donor materials
3.2 D18 series random polymer donor materials
3.3 PTs series random polymer donor materials
3.4 PTB and PCE11 series random polymer donor materials
3.5 J series random polymer donor materials
4 Random polymer acceptors
4.1 NDI series random polymer acceptor materials
4.2 BTI series random polymer acceptor materials
4.3 PSMA series random polymer acceptor materials
5 Application of random copolymerization to OSCs stability
6 Conclusion and outlook
Chunyan Li , Xin Lin , Wen Wang , Hongyu Zhen . Ternary Random Strategy Applied in Active Layer of High Performance Non-Fullerene Organic Solar Cells[J]. Progress in Chemistry, 2024 , 36(9) : 1316 -1335 . DOI: 10.7536/PC240204
表1 Performance Parameters of Organic Solar Cells Based on PBDB Series Random Polymer DonorsTable 1 Device performance of organic solar cells based on PBDB series random polymer donors |
| Active layer | VOC /V | JSC /mA·cm-2 | FF /% | PCE /% | Ref |
|---|---|---|---|---|---|
| T1:IT-4F | 0.899 | 21.5 | 78 | 15.1 | 10 |
| S1:Y6 | 0.877 | 25.402 | 73.7 | 16.421 | 11 |
| PBDB-TF-T10:Y6 | 0.84 | 27.9 | 70 | 16.4 | 12 |
| PM6:S3:Y6 | 0.856 | 25.86 | 79.17 | 17.53 | 13 |
| L1:L8-BO | 0.901 | 26.38 | 78.9 | 18.75 | 14 |
| L2:L8-BO | 0.898 | 26.26 | 78.6 | 18.54 | 14 |
| PF1:Y6 | 0.87 | 26.3 | 76 | 17.3 | 15 |
| PF2:Y6 | 0.88 | 22.9 | 70 | 14.1 | 15 |
| PM6-TTO-10:BTP-eC9 | 0.860 | 26.80 | 79.97 | 18.37 | 16 |
| PTF3:Y6-BO | 0.83 | 26.5 | 74.0 | 16.3 | 17 |
| PTF5:Y6-BO | 0.84 | 28.0 | 77.2 | 18.2 | 17 |
| PTF10:Y6-BO | 0.84 | 27.0 | 75.1 | 17.0 | 17 |
| PM1:Y6 | 0.87 | 25.9 | 78 | 17.6 | 18 |
| PM2:Y6 | 0.90 | 24.9 | 69 | 15.5 | 18 |
| PM6-Tz20:Y6 | 0.86 | 27.3 | 75 | 17.6 | 19 |
| PM6-Tz40:Y6 | 0.85 | 25.3 | 72 | 15.5 | 19 |
| OPz1:Y6 | 0.871 | 25.34 | 73.7 | 16.28 | 20 |
| OPz2:Y6 | 0.871 | 24.81 | 67.1 | 14.54 | 20 |
| OPz3:Y6 | 0.881 | 18.68 | 43.1 | 7.09 | 20 |
| PMZ-10:Y6 | 0.834 | 27.96 | 78.2 | 18.23 | 21 |
| PMZ-20:Y6 | 0.841 | 27.39 | 76.6 | 17.64 | 21 |
| PMZ-30:Y6 | 0.847 | 26.92 | 75.3 | 17.17 | 21 |
| PMZ1:Y6 | 0.841 | 26.1 | 75.5 | 16.6 | 22 |
| PMZ2:Y6 | 0.854 | 26.9 | 77.2 | 17.8 | 22 |
| PMZ3:Y6 | 0.857 | 26.1 | 75.5 | 16.9 | 22 |
| PM6-SiCl-10%:Y6 | 0.870 | 25.37 | 73.48 | 16.22 | 23 |
| PM6-SiCl-15%:Y6 | 0.875 | 25.26 | 70.03 | 15.48 | 23 |
| PM6-SiCl-20%:Y6 | 0.881 | 24.88 | 67.24 | 14.73 | 23 |
| PBNB80:BO-4Cl | 0.850 | 25.7 | 73.3 | 16.0 | 24 |
| PM6-5Si:N3 | 0.852 | 26.32 | 75.81 | 17.01 | 25 |
| PQB-2:PY-IT | 0.942 | 24.2 | 79.5 | 18.1 | 26 |
| PM6-C5:Y7 | 0.86 | 26.3 | 72 | 16.7 | 27 |
| PM6-C10:Y7 | 0.86 | 26.8 | 74 | 17.0 | 27 |
| PM6-C20:Y7 | 0.86 | 24.7 | 70 | 14.9 | 27 |
| PM6-C30:Y7 | 0.84 | 23.9 | 68 | 13.8 | 27 |
| PM6-TzBI-5:L8-BO | 0.894 | 25.45 | 78.10 | 17.77 | 28 |
| PM6-TzBI-10:L8-BO | 0.897 | 25.63 | 79.84 | 18.36 | 28 |
| PM6-TzBI-15:L8-BO | 0.905 | 25.40 | 77.67 | 17.85 | 28 |
| PM6-TzBI-20:L8-BO | 0.909 | 25.03 | 76.78 | 17.47 | 28 |
| PNB-1:L8-BO | 0.903 | 26.21 | 78.04 | 18.47 | 29 |
| PNB-3:L8-BO | 0.907 | 26.59 | 78.86 | 19.02 | 29 |
| PNB-5:L8-BO | 0.914 | 25.53 | 76.20 | 17.78 | 29 |
| PM6-Ir0.5:Y6 | 0.845 | 26.28 | 74.02 | 16.44 | 30 |
| PM6-Ir1:Y6 | 0.842 | 26.12 | 78.41 | 17.24 | 30 |
| PM6-Ir2.5:Y6 | 0.847 | 26.07 | 74.15 | 16.37 | 30 |
| PM6-Ir5:Y6 | 0.842 | 25.92 | 73.43 | 16.03 | 30 |
图4 PBDB系列非等量无规聚合物给体Fig.4 The structure of PBDB series non-equivalent random polymer donors |
表2 Performance parameters of organic solar cell devices based on PBDB series non-equivalent random polymer donor materialsTable 2 Device performance of organic solar cells based on PBDB series non-equivalent random polymer donors |
| Active layer | VOC /V | JSC /mA·cm-2 | FF /% | PCE /% | Ref |
|---|---|---|---|---|---|
| PM6-D1:Y6 | 0.85 | 26.47 | 78.7 | 17.71 | 31 |
| PM6-D2:Y6 | 0.85 | 26.18 | 75.6 | 16.82 | 31 |
| PM6-D3:Y6 | 0.86 | 26.01 | 76.1 | 17.02 | 31 |
| DM1:Y6 | 0.85 | 25.86 | 78.29 | 17.21 | 32 |
| DM2:Y6 | 0.86 | 25.63 | 75.48 | 16.65 | 32 |
| PM6-T5:Y6 | 0.89 | 23.20 | 68.61 | 14.17 | 34 |
| PM6-T10:Y6 | 0.87 | 26.43 | 66.53 | 15.31 | 34 |
| PM6-T15:Y6 | 0.86 | 27.02 | 71.14 | 16.61 | 34 |
| PM6-T20:Y6 | 0.88 | 24.12 | 65.41 | 13.94 | 34 |
| PM6-A5:Y6 | 0.84 | 26.36 | 68.65 | 15.20 | 33 |
| PM6-A15:Y6 | 0.83 | 23.95 | 62.49 | 12.42 | 33 |
| PM6-O5 | 0.84 | 27.87 | 75.39 | 17.65 | 35 |
| PM6-O10 | 0.84 | 26.23 | 69.69 | 15.34 | 35 |
| PM6-O30 | 0.84 | 19.09 | 62.66 | 10.14 | 35 |
图5 (1)D18系列无规聚合物给体,(2)聚噻吩系列无规聚合物给体,(3)PTB系列和PCE11系列无规聚合物给体,(4)J系列无规聚合物给体Fig. 5 (1)The structure of D18 series random polymer donors, (2) The structure of PTs series random polymer donors, (3) The structure of PTB and PCE11series random polymer donors, (4) The structure of J series random polymer donors |
表3 Performance parameters of organic solar cell devices with other random polymer donorsTable 3 Device performance of organic solar cells based on other series random copolymer donors |
| Active layer | VOC /V | JSC /mA·cm-2 | FF /% | PCE /% | Ref |
|---|---|---|---|---|---|
| D18-20%Cl:Y6 | 0.861 | 27.20 | 78.06 | 18.28 | 37 |
| D18-40%Cl:Y6 | 0.866 | 26.34 | 75.46 | 17.21 | 37 |
| OPz11:Y6 | 0.865 | 27.02 | 78.71 | 18.42 | 38 |
| OPz12:Y6 | 871 | 26.20 | 73.82 | 16.85 | 38 |
| PW2:BTP-ec9-4F | 0.87 | 27.32 | 72.72 | 17.19 | 40 |
| PDCBT-Cl:ITIC-Th1 | 0.93 | 17.91 | 68.2 | 11.33 | 41 |
| PDCBT-Cl-Si5:ITIC-Th1 | 0.93 | 18.70 | 70.1 | 12.67 | 41 |
| PDCBT-Cl-Si10:ITIC-Th1 | 0.92 | 18.28 | 69.9 | 11.79 | 41 |
| PDCBT-Cl-Si15:ITIC-Th1 | 0.91 | 17.13 | 64.0 | 9.95 | 41 |
| P5TCN-F0:Y6 | 0.81 | 20.83 | 68.8 | 11.5 | 42 |
| P5TCN-F25:Y6 | 0.79 | 27.13 | 77.1 | 16.6 | 42 |
| P5TCN-F35:Y6 | 0.80 | 26.87 | 75.0 | 16.1 | 42 |
| P5TCN-F50:Y6 | 0.82 | 24.78 | 72.0 | 14.6 | 42 |
| P5TCN-F25:Y6:PCBM | 0.80 | 27.55 | 77.7 | 17.2 | 42 |
| PCE10-BDT2F-0.3:Y6 | 0.678 | 26.10 | 66.56 | 11.76 | 44 |
| PCE10-BDT2F-0.5:Y6 | 0.720 | 26.01 | 69.42 | 13.01 | 44 |
| PCE10-BDT2F-0.6:Y6 | 0.739 | 26.01 | 64.08 | 12.36 | 44 |
| PCE10-BDT2F-0.7:Y6 | 0.746 | 26.17 | 68.95 | 13.46 | 44 |
| PCE10-BDT2F-0.8:Y6 | 0.753 | 26.36 | 69.45 | 13.80 | 44 |
| PCE10-BDT2F-0.9:Y6 | 0.766 | 25.14 | 67.71 | 13.03 | 44 |
| PCE10-BDT2Cl-0.3:Y6 | 0.700 | 23.56 | 66.24 | 10.92 | 44 |
| PCE10-BDT2Cl-0.5:Y6 | 0.724 | 25.37 | 64.41 | 11.83 | 44 |
| PCE10-BDT2Cl-0.7:Y6 | 0.743 | 24.85 | 65.58 | 12.09 | 44 |
| Si25:Y14 | 0.782 | 26.82 | 73.38 | 15.39 | 46 |
| TBFCl50-FTAZ:ITIC | 0.876 | 18.45 | 73.8 | 11.94 | 48 |
| TBFCI50-BDD:ITIC | 0.961 | 18.17 | 71.3 | 12.46 | 48 |
| TBFCI50-BDD:IDIC-C4ph | 0.908 | 17.66 | 76.6 | 12.29 | 48 |
| PBTA-PS-F:ITIC | 0.97 | 18.46 | 75.29 | 13.48 | 49 |
| PSBTZ-60:ITIC | 0.91 | 18.0 | 62.7 | 10.3 | 50 |
表4 Performance parameters of organic solar cell devices based on random polymer acceptors of NDI and BTI seriesTable 4 Device performance of organic solar cells based on NDI and BTI series random polymer acceptors |
| Active layer | VOC /V | JSC /mA·cm-2 | FF /% | PCE /% | Ref |
|---|---|---|---|---|---|
| copolymer2:PTB7-Th | 0.805 | 9.60 | 45.1 | 3.48 | 52 |
| copolymer3:PTB7-Th | 0.790 | 12.66 | 48.6 | 4.86 | 52 |
| copolymer4:PTB7-Th | 0.794 | 13.75 | 48.3 | 5.27 | 52 |
| PNDI-2FT-0.1:PBDB-T | 0.850 | 16.62 | 67.1 | 9.46 | 53 |
| PNDI-2FT-0.2:PBDB-T | 0.848 | 14.66 | 64.4 | 8.05 | 53 |
| PNDI-2FT-0.3:PBDB-T | 0.823 | 11.91 | 60.7 | 5.85 | 53 |
| 0.3-NDI:PBDT(T)TPD | 1.04 | 7.4 | 59.1 | 4.6 | 54 |
| 0.5-NDI:PBDT(T)TPD | 1.05 | 9.1 | 56.1 | 5.4 | 54 |
| 0.7-NDI:PBDT(T)TPD | 1.04 | 4.1 | 48.3 | 2.1 | 54 |
| BSS10:PBDB-T | 0.86 | 18.55 | 64 | 10.1 | 55 |
| BSS20:PBDB-T | 0.86 | 17.07 | 65 | 9.58 | 55 |
| PNDI-2T-TR(5):PBDB-T | 0.86 | 14.34 | 64.32 | 8.13 | 56 |
| PNDI-2T-TR(10):PBDB-T | 0.80 | 11.36 | 53.53 | 4.89 | 56 |
| PNDI-2FT-TR10:PM6 | 0.921 | 17.32 | 67 | 10.71 | 57 |
| PNDI-2FT-TR20:PM6 | 0.913 | 14.25 | 62 | 8.07 | 57 |
| NOE10:PBDT-TAZ | 0.84 | 12.9 | 75 | 8.1 | 58 |
| NOE20:PBDT-TAZ | 0.83 | 11.9 | 73 | 7.3 | 58 |
| NOE30:PBDT-TAZ | 0.82 | 10.7 | 69 | 6.1 | 58 |
| BTI2-10TPD:PTB7-Th | 1.04 | 12.72 | 56.25 | 7.42 | 59 |
| BTI2-30TPD:PTB7-Th | 1.05 | 13.56 | 58.25 | 8.28 | 59 |
| BTI2-50TPD:PTB7-Th | 1.07 | 12.23 | 49.95 | 6.54 | 59 |
| BTI2-30BT:PTB7-Th | 0.99 | 12.11 | 45.80 | 5.48 | 60 |
| BTI2-30PhI:PTB7-Th | 1.08 | 12.91 | 48.75 | 6.80 | 60 |
| PBTI2(50DT)-FT:PTB7-Th | 1.01 | 13.96 | 59.0 | 8.32 | 61 |
表5 Performance parameters of organic solar cell devices based on PSMA series random polymer acceptor materialsTable 5 Device performance of organic solar cells based on PSMA series random copolymer acceptor materials |
| Active layer | VOC /V | JSC /mA·cm-2 | FF /% | PCE/% | Ref | |
|---|---|---|---|---|---|---|
| PTPBT-ET0.1:PBDB-T | 0.855 | 19.48 | 62.3 | 10.37 | 63 | |
| PTPBT-ET0.2:PBDB-T | 0.864 | 20.78 | 65.5 | 11.76 | 63 | |
| PTPBT-ET0.3:PBDB-T | 0.899 | 21.33 | 65.3 | 12.52 | 63 | |
| PTPBT-ET0.4:PBDB-T | 0.900 | 21.22 | 55.3 | 10.56 | 63 | |
| PTPBT-ET0.5:PBDB-T | 0.902 | 18.77 | 57.5 | 9.73 | 63 | |
| PTPBT-ET0.75:PBDB-T | 0.903 | 14.50 | 38.8 | 5.08 | 63 | |
| PYE10:PBDB-T | 0.900 | 20.93 | 69.43 | 13.09 | 64 | |
| PYE20:PBDB-T | 0.905 | 20.97 | 71.63 | 13.60 | 64 | |
| PYE30:PBDB-T | 0.875 | 21.63 | 70.18 | 13.28 | 64 | |
| PYE40:PBDB-T | 0.861 | 22.24 | 68.12 | 13.04 | 64 | |
| PYT-TOE(10): PBDB-T | 0.91 | 21.73 | 64.63 | 12.77 | 65 | |
| PYT-TOE(20):PBDB-T | 0.91 | 18.75 | 61.41 | 10.49 | 65 | |
| PYT-TOE(30):PBDB-T | 0.89 | 16.74 | 55.01 | 8.16 | 65 | |
| PYTS-0.1:PBDB-T | 0.92 | 22.52 | 68 | 14.19 | 66 | |
| PYTS-0.3:PBDB-T | 0.92 | 22.91 | 70 | 14.68 | 66 | |
| PYTS-0.5:PBDB-T | 0.92 | 14.46 | 60 | 7.91 | 66 | |
| PYSe-TCl10:PBDB-T | 0.894 | 22.84 | 64.3 | 13.23 | 67 | |
| PYSe-TCl20:PBDB-T | 0.902 | 23.08 | 68.3 | 14.21 | 67 | |
| PYSe-TCl30:PBDB-T | 0.911 | 21.02 | 61.4 | 11.85 | 67 | |
| PY-91:PM6 | 0.949 | 23.66 | 73.9 | 16.59 | 68 | |
| PY-82:PM6 | 0.950 | 23.82 | 75.8 | 17.15 | 68 | |
| PY-73:PM6 | 0.951 | 23.53 | 75.0 | 16.78 | 68 | |
| PA1-o:PM6 | 0.925 | 23.99 | 72.2 | 16.0 | 69 | |
| PYTH-A:PM6 | 0.943 | 22.89 | 71.28 | 15.39 | 70 | |
| PYTCl-B:PM6 | 0.942 | 23.49 | 73.04 | 16.16 | 70 | |
| PYTH-A:PM6 | 0.937 | 20.35 | 68.78 | 13.12 | 70 | |
| PYTCl-B:PM6 | 0.935 | 20.76 | 70.14 | 13.61 | 70 | |
| PY-0.1OBO:PM6 | 0.967 | 24.4 | 67.0 | 15.9 | 71 | |
| PY-0.2OBO:PM6 | 0.972 | 25.1 | 67.9 | 16.7 | 71 | |
| PY-0.3OBO:PM6 | 0.977 | 24.1 | 68.1 | 15.9 | 71 | |
| PY-0.5OBO:PM6 | 0.991 | 19.1 | 69.1 | 13.2 | 71 | |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
/
| 〈 |
|
〉 |