

Iron-Blocking Antibacterial Agents Based on Gallium Salts or Gallium Porphyrins
Received date: 2024-01-11
Revised date: 2024-05-17
Online published: 2024-07-01
Supported by
National Natural Science Foundation of China(21961132005)
Innovation and Creation Project of China Oilfield Service Co., LTD(HYZC-3-20240329-41577)
Interfering with bacterial iron metabolism is a non-antibiotic antibacterial strategy that is not easy to cause bacterial resistance.in this review,firstly,an iron-blocking antibacterial therapy which can kill drug-resistant bacteria but not easily cause drug resistance is introduced.Then,the mechanism of iron-blocking antibacterial therapy is explained from the iron uptake pathway and heme uptake pathway of bacteria.in addition,the types,in vitro antibacterial properties,and In vivo therapeutic effects of the iron blocking antimicrobials based on gallium salts and gallium porphyrins are reviewed in detail.Finally,the future development and practical application of the antimicrobials based on iron-blocking mechanism are prospected。
1 Introduction
2 Iron-blocking antibacterial mechanism
2.1 Host iron restriction for bacteria
2.2 Iron acquisition by bacteria
2.3 Iron-blocking antibacterial mechanism of gallium ion
2.4 Iron-blocking antibacterial mechanism of gallium porphyrins
3 Iron-blocking agents
3.1 Gallium salts
3.2 Gallium porphyrins
3.3 Combination of gallium ions and gallium porphyrins
4 Conclusion and outlook
Hao Zhang , Lei Zhang , Zhiyong Wang , Xinyu Zhou , Ruixiao Gao . Iron-Blocking Antibacterial Agents Based on Gallium Salts or Gallium Porphyrins[J]. Progress in Chemistry, 2024 , 36(9) : 1392 -1400 . DOI: 10.7536/PC240106
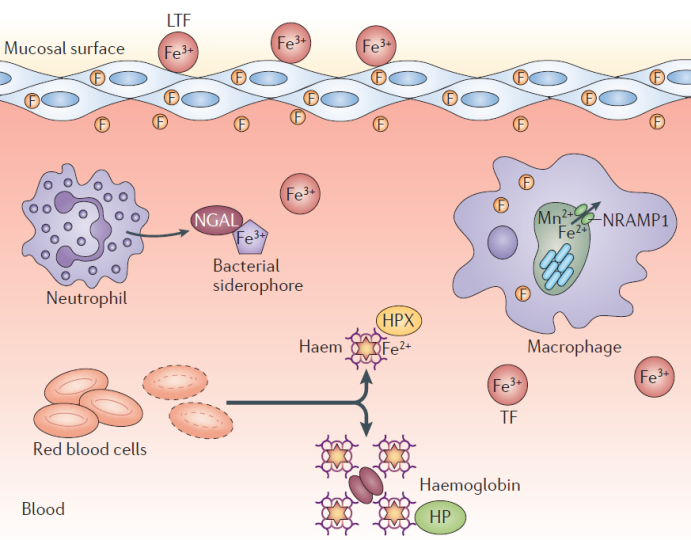
图2 细菌感染时铁的获取方式。CM,细胞质膜;HO1,血红素加氧酶1;IM,内膜;Isd,铁调节表面因子;OM,外膜;SIP,铁蛋白相互作用蛋白;TF,转铁蛋白[6]Fig. 2 Iron acquisition during bacterial infections. CM, cytoplasmic membrane; HO1, haem oxygenase 1; IM, inner membrane; Isd, Fe‑regulated surface determinant; OM, outer membrane; SIP, siderophore-interacting protein; TF, transferrin [6] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
/
| 〈 |
|
〉 |