

Dissolved Aluminum Measurement Methods and Their Application in Atmospheric Aerosol Research
Received date: 2024-02-19
Revised date: 2024-06-05
Online published: 2024-08-30
Supported by
National Natural Science Foundation of China(42277088)
Guangdong Basic and Applied Basic Research Foundation(2022A1515110371)
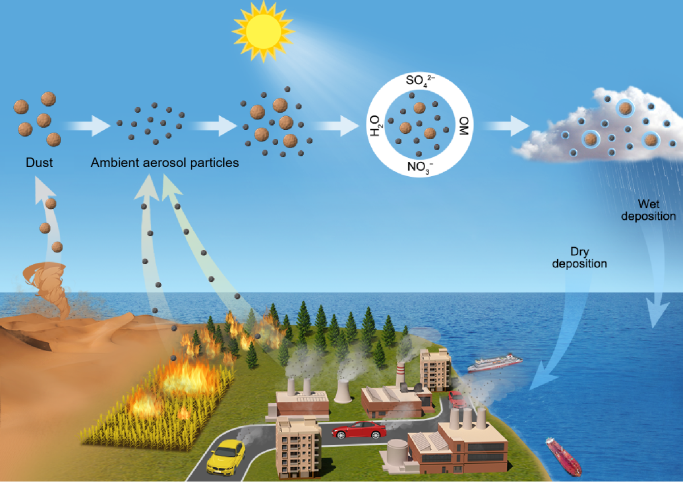
Atmospheric deposition of desert dust aerosol is a major source of key nutrients for surface seawater In open oceans,significantly impacting marine biogeochemistry and primary productivity.As a tracer for desert dust aerosol,aluminum(Al)is widely used to estimate deposition fluxes of desert dust aerosol into the oceans,and dissolved Al concentrations in surface seawater and aerosol particles are key parameters for using this method to estimate desert dust deposition fluxes into the oceans.in this paper,we first review separation,extraction and detection methods used to measure dissolved Al in seawater and aerosol samples,and discuss their principles,advantages,limitations and applicability.After advances in aerosol Al solubility are systematically reviewed,we point out that the uncertainties in aerosol Al solubility are the bottleneck which currently limits accurate estimations of desert dust deposition fluxes into the oceans,and further analyze the sources of these uncertainties.in the final,we also outline research directions for dissolved Al analysis and aerosol Al solubility research。
1 Introduction
2 Pretreatment methods for dissolved aluminum
2.1 Filtration
2.2 Separation and preconcentration methods for seawater samples
2.3 Extraction methods for soil and aerosol samples
3 Detection methods for dissolved aluminum
3.1 UV-Visible spectrophotometry
3.2 Fluorescence spectrophotometry
3.3 Atomic spectrometry
3.4 Inductively coupled plasma mass spectrometry
4 Research progress of aerosol aluminum solubility
5 Conclusion and outlook
Tianyu Zhang , Zhenming Zhu , Fu Wang , Lanxiadi Chen , Rui Li , Mingjin Tang . Dissolved Aluminum Measurement Methods and Their Application in Atmospheric Aerosol Research[J]. Progress in Chemistry, 2024 , 36(9) : 1401 -1411 . DOI: 10.7536/PC240205
表1 Separation and Enrichment of Soluble Aluminum in SeawaterTable 1 Separation and preconcentration method for dissolved aluminum in seawaters |
| Methods | Advantages | Drawbacks |
|---|---|---|
| SPE | High level of automation. Low risk of contamination. | High technical experience when combined with FIA |
| LLE | Easy to operate. | Long extraction time Small extraction efficiency Labor intensive |
| DLLME | Short extraction time. Extractant is easy to collect. | Not robust enough to be completely automated. |
| CPE | Less laboratory wastes. | Mostly off-line Labor intensive |
| HPLC | High sensitivity. Suitable for Al species separated. | Mostly off-line High instrument requirements |
表2 Application of Ultraviolet-Visible Spectrophotometry in EnvironmentTable 2 Method for determination of dissolved aluminum based on UV-Visible Spectrophotometry |
| Colour-forming system | Detection limit/nmol/L | Linear range/µmol/L | Sample matrix | Ref |
|---|---|---|---|---|
| Al-Aluminon | 480 | 29.63~137.04 | well and pond water | 55 |
| Al-CAS | 0.8 | 0.001~0.25 | seawater | 54 |
| Al-ECR | 5.19 | 0.15~14.81 | waste and tap water | 56 |
| Al-ECR | 74 | 2.78~23.15 | surface, drinking water, and tap water | 57 |
| Al-ECR | 11.11 | - | soil extracts and ground waters | 58 |
| Al-Quercetin | 50 | 0.1 ~ 80 | river and stream water | 59 |
| Al-Quercetin | 260 | 0.74~18.52 | tap water | 60 |
| Al-Morin | 160 | 0.1~0.8 | - | 61 |
| Al-Morin | - | 0.37~185.19 | natural water(e.g. river, pond and seawater) | 62 |
表3 Observations of aluminium solubility in aerosols over parts of the globeTable 3 Distribution of Al solubility in atmospheric aerosols in global distinct regions |
| Location | Leaching solution | Leaching technique | Contact time | Detection method | Sample | Al solubility |
|---|---|---|---|---|---|---|
| Pacific Ocean (Hawaii) [53] | Seawater | Batch (shaking) | 5 min | FIA | TSP | 0.087%~14.3% |
| Pacific Ocean[88] | Deionized water | Flow-through | Instant | HR-ICP-MS | TSP | 0.2%~15.9% |
| North Atlantic Ocean[89] | Deionized water | Flow-through | Instant | HR-ICP-MS | TSP | 4%~50% |
| North Atlantic Ocean[75] | Deionized water | Flow-through | Instant | ICP-MS | TSP | 0.34%~28% |
| Acetic acid | Batch (soaking) | 10 min | 4.1%~100% | |||
| North East Atlantic Ocean[49] | Ammonium acetate | Batch (shaking) | 60~120 min | ICP-OES and ICP-MS | dp>1.09 μm | 0.43%~2.03% |
| dp<1.09 μm | 4.53%~10.31% | |||||
| South East Atlantic Ocean[71] | Ammonium acetate | Batch (shaking) | 60 min | ICP-OES | TSP | 3%~78% |
| PM1 | 7%~79% | |||||
| East China Sea (Huaniao Island) [82] | Deionized water | Batch (sonication) | 40 min | ICP-OES | TSP | 0.1%~23% |
| East China Sea[81] | Deionized water | Batch (shaking) | 60 min | ICP-OES | TSP | 5.1% ± 2.8% (non-dust period) |
| 1.1 %± 1.8% (dust period) | ||||||
| Qingdao, China[90] | Deionized water | Batch (shaking) | 30 min | ICP-MS | TSP | 2.8% ± 3.9% |
| Hiroshima, Japan[50] | Deionized water | Batch (shaking) | 30 min | ICP-MS | TSP | 1.46%~7.39% |
| dp>1.3 μm | 3.25% ± 3.41% | |||||
| dp<1.3 μm | 8.82% ± 6.48% |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
蔺庆伟, 马剑敏, 彭雪, 孙健, 刘碧云, 吴振斌. 生态环境学报, 2019, 28(9): 1915.
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
Menzel Barraqueta J L,
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
(孙梅, 刘桂建, 吴强华, 环境化学, 2013, 32(06): 1016.).
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
(方宏达, 陈锦芳, 段金明, 陈进生, 林清杰, 陈少华. 生态环境学报, 2015, 24(11): 1872.).
|
| [49] |
|
| [50] |
|
| [51] |
(薛金林, 任景玲, 海洋环境科学, 2019, 38(06): 945. ).
|
| [52] |
(常燕, 冯冲, 瞿建国, 张经, 环境科学, 2015, 36(04): 1164.).
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
(赵君威, 梅坛, 鄢国强, 陶美娟, 陈忠颖. 理化检验-化学分册, 2013, 49(3): 364.).
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
(齐宇轩, 周杨. 海洋气象学报, 2021, 41(2): 1. ).
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
(朱敏, 石金辉, 贲孝宇, 仇帅, 高会旺, 姚小红, 中国环境科学, 2016, 36(11): 3245.)
|
/
| 〈 |
|
〉 |