

Key Environmental Behaviors of Tire Wear Particles and Their Influencing Mechanisms
Received date: 2024-07-10
Revised date: 2024-11-07
Online published: 2025-01-07
Supported by
National Natural Science Foundation of China(22241602)
National Natural Science Foundation of China(22125603)
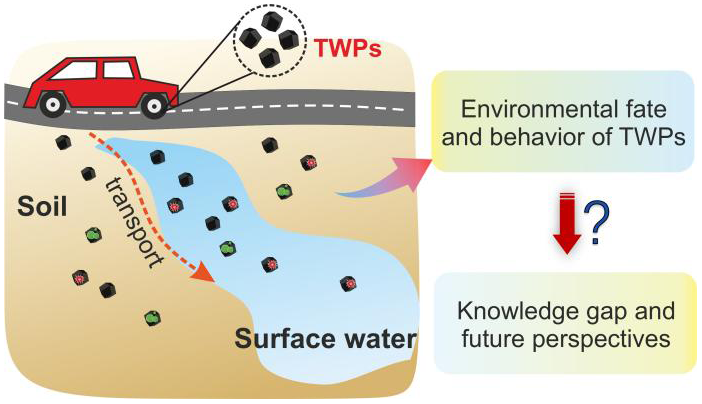
Due to the rapid growth in the number of vehicles and freight transport, tire wear particles (TWPs), generated from the friction between tires and road surfaces, have become the main source of microplastics in the environment. TWPs are widely detected in various environmental media, including soil, surface water, and sediments. An in-depth mechanistic understanding of the environmental interfacial processes of TWPs is of great significance for the control of microplastic pollution. In this paper, we first summarized recent progress in the interfacial chemical processes of TWPs, including the transport behavior, environmental transformation, release of toxic additives, and the adsorption of co-existing pollutants on TWPs. We then addressed some existing issues in current research and proposed future directions toward a better understanding of the environmental behavior and potential environmental risks of TWPs.
1 Introduction
2 Fate and transport of TWPs in the environment
2.1 Transport via rainfall and runoff
2.2 Atmospheric transport
2.3 Aggregation and sedimentation behavior of TWPs in the aquatic environment
3 Transformations of TWPs in the environment
3.1 Physical and chemical transformations of TWPs
3.2 Microbial transformation of TWPs
4 Release of additives from TWPs
5 Accumulation of contaminants on TWPs
6 Conclusion and outlook
Hongwei Liu , Yuxin Yuan , Tianchi Cao , Tong Zhang , Wei Chen . Key Environmental Behaviors of Tire Wear Particles and Their Influencing Mechanisms[J]. Progress in Chemistry, 2025 , 37(1) : 103 -111 . DOI: 10.7536/PC240708
| [1] |
|
| [2] |
|
| [3] |
(杨杰, 李连祯, 周倩, 李瑞杰, 涂晨, 骆永明. 土壤学报, 2021, 58(2):281.).
|
| [4] |
|
| [5] |
(焦萌, 曹秉帝, 张涛. 环境科学学报, 2020, 40(12): 16.).
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
(陈瑶, 刘金, 张颖昕, 李佳旸, 李桂菊. 应用生态学报, 2022, 33 (8): 2262.).
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
(吴琳, 张新峰, 门正宇, 张静, 常俊雨, 张宝玺, 毛洪钧. 中国环境科学, 2020, 40(4): 1486.).
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
(范秀磊, 邹晔锋, 刘加强, 李莹, 刘强, 侯俊. 环境科学, 2021, 42 (4):1901.).
|
| [87] |
|
| [88] |
|
| [89] |
|
/
| 〈 |
|
〉 |