

The Impact of Aging on the Physicochemical Properties, Environmental Processes and Toxic Effects of Microplastics
Received date: 2024-07-10
Revised date: 2024-11-12
Online published: 2024-11-12
Supported by
National Natural Science Foundation of China(22241604)
National Natural Science Foundation of China(22125606)
Microplastic pollution has become a major environmental issue of global concern. Microplastics can undergo aging under various environmental conditions. The aging process will change the physical and chemical properties of microplastics, thereby leading to changes in their environmental behaviors and toxicities. Therefore, exploring the aging process and mechanism of microplastics is of significance for understanding the environmental processes and health risks of microplastics. This article focuses on the aging process of microplastics in the environment and reviews it from the aspects of aging pathways, influencing factors, interactions with pollutants, release of chemical substances, and changes in toxicities. It also looks forward to the existing challenges and future research directions in the current studies on microplastic aging.
1 Introduction
2 Pathways of microplastics aging
2.1 Physical aging of microplastics
2.2 Chemical aging of microplastics
2.3 Biological aging of microplastics
2.4 Artificial aging of microplastics
3 The factors influencing microplastics aging
3.1 The impact of physical and chemical properties on microplastics aging
3.2 The impact of environmental conditions on microplastics aging
4 The impact of aging on microplastics
4.1 The impact of aging on the physical and chemical properties of microplastics
4.2 The impact of aging on the interaction between microplastics and pollutants
4.3 The impact of aging on the release of chemicals from microplastics
4.4 The impact of aging on the toxicities of microplastics
5 Conclusions and perspectives
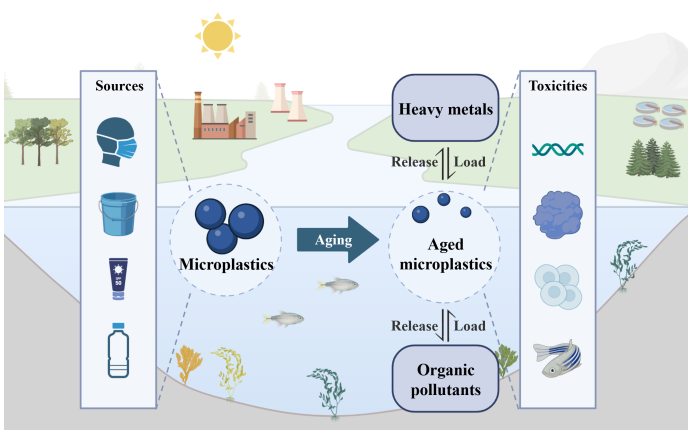
Yulong Wang , Yue Li , Fengbang Wang , Maoyong Song . The Impact of Aging on the Physicochemical Properties, Environmental Processes and Toxic Effects of Microplastics[J]. Progress in Chemistry, 2025 , 37(1) : 46 -58 . DOI: 10.7536/PC240707
Table 1 Influence of environmental factors on the aging of microplasticsTable 1 The effects of environmental factors on the aging of microplastics |
| Microplastics | Aging pathway | Factor | Effect | Ref | ||
|---|---|---|---|---|---|---|
| PVC | Light | Wave length | UVC > UVA and UVB | 81 | ||
| PS | Sediment | O2 | Alternating anoxic-oxic conditions > static oxic and static anoxic conditions | 83 | ||
| PET | Dark and anoxic conditions | Reducing agents | Reducing agents: (+) a | 86 | ||
| PP | Activated persulfate and hydrothermal treatment | Temperature | High temperature: (+) | 89 | ||
| PS | Compost | Temperature | High temperature: (+) | 90 | ||
| PS | Light | HA | HA: (+) | 91 | ||
| PS | Light and HA | Salinity | Hight salinity: (+) | 91 | ||
| PS | Light | Ions | NO3-,Cl-,and Br-: (+); HCO3-: (/) b | 92 | ||
| PBAT | Light | Ions | NO3-: (+) Cl- and Br-: (-) c | 93 | ||
| PP | Light | Ions | Cl-: (-) | 94 | ||
| PE | Soil burial | pH | High pH: (+) | 95 | ||
| PE | Seawater aquarium | Temperature and pH | High temperature: (+); Low pH: (+) | 96 | ||
| PP and EVA d | Light | pH | Low pH: (+) | 97 | ||
| EPS e | Light | pH | Low pH: (/) | 97 | ||
| PS | Light | Organic matter | Hyaluronic acid and vitamin C: (+) | 98 | ||
| PP | Light | Organic matter | HA and FA: (-) | 99 | ||
| PS | Light | Organic matter | FA<1kDa: (+) | 100 | ||
| PE f | Light | Organic matter | Paraquat and Mancozeb: (+); Chlorpyrifos and Sulfur: (/) | 101 | ||
| PS | Light | Organic matter | Pyrite: (+) | 102 | ||
| PET | Light | Soil component | Clay,iron oxides,MnO2,SiO2,and Al2O3: (+); Organic carbon: (-) | 103 | ||
| PVC and PET | Light | Organic matter | Kaolinite and montmorillonite: (+) | 104 | ||
| Note: a(+): Promotion; b(/): No significant effect; c(-): Inhibition; dEVA: ethylene-vinyl acetate; eEPS: expanded polystyrene; f: PE film | ||||||
Figure 4 During the aging process of microplastics, the microplastics themselves, the environmental pollutants loaded by the microplastics, and the released additives and intermediates will produce different biological toxicityFig. 4 During the aging process of microplastics, microplastics themselves,environmental pollutants loaded on microplastics, and released additives and intermediates can produce different biotoxicities |
| [1] |
Organization for Economic Co-operation and Development (OECD). Global Plastics Outlook: Policy Scenarios to 2060, OECD Publishing, Paris, 2022.
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
Campanale, Massarelli, Savino, Locaputo, Uricchio. Int. J. Environ. Res. Public. Health, 2020, 17: 1212.
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
/
| 〈 |
|
〉 |