

High-Voltage Tolerant Electrolyte for Lithium-Ion Batteries
Received date: 2024-03-06
Revised date: 2024-05-06
Online published: 2024-07-01
Supported by
National Natural Science Foundation of China(U22A20419)
National Natural Science Foundation of China(51971094)
Guangdong Provincial Key Research and Development Program(2023B0909060003)
Guangdong Provincial Key Research and Development Program(2020B0909040003)
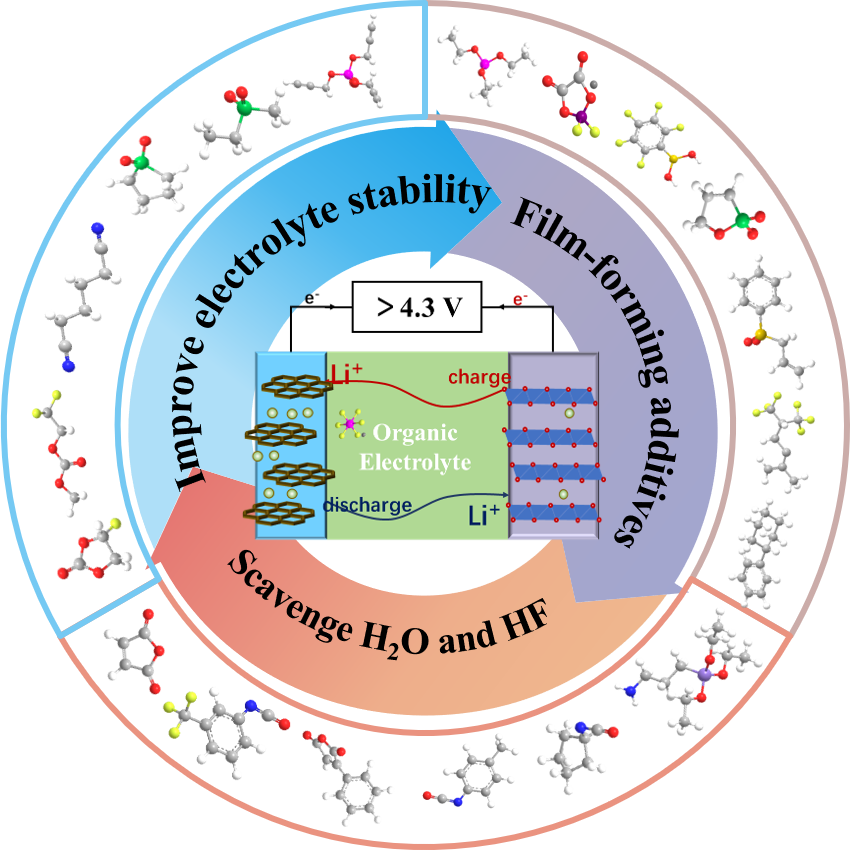
With the rapid development of consumer intelligent electronic devices and electric vehicles, the development of lithium-ion batteries with high energy density has become a very urgent and important issue. Using high-voltage electrode materials and enhancing the work voltage of batteries is an effective pathway to realize the high energy density of battery. However, the conventional carbonate-based electrolyte will undergo oxidation reactions when the voltage is higher than 4.3 V, which will lead to electrolyte decomposition, and finally resulting in the failure of the battery. Actually, it has become one of the main bottlenecks in the development of high-voltage batteries. In order to solve this problem, researchers have carried out a lot of exploration in the design of high-voltage electrolyte in recent years, and made many important research achievements. This review introduces the failure mechanism of batteries under high voltage, and focuses on the strategies and research progress in suppressing high voltage failure from the perspective of electrolytes in recent years, indicates the challenges still existing in the design of high-voltage electrolyte, and finally prospects the future developments of high voltage lithium-ion battery electrolyte.
Contents
1 Introduction
2 Failure mechanism of high-voltage batteries
2.1 Electrolyte decomposition
2.2 Transition metal ion leaching
2.3 HF erosion
3 Progress on high-voltage electrolyte
3.1 Improvement of intrinsic stability of electrolyte
3.2 Construction of stable CEI Layer
3.3 Scavenge H2O and HF
4 Conclusion and outlook
Luoqian Li , Mumin Rao , Hong Chen , Shijun Liao . High-Voltage Tolerant Electrolyte for Lithium-Ion Batteries[J]. Progress in Chemistry, 2024 , 36(10) : 1456 -1472 . DOI: 10.7536/PC240310
图2 (a)不同阴极电池100圈循环后石墨阳极表面过渡金属离子含量[12];(b)LiCoO2在不同电压下的滴定质谱和在线电化学质谱[21];(c)EC的化学氧化机理[8]Fig. 2 (a) Transition metal ion content on the surface of graphite anode after 100 cycles of different cathode batteries[12]. (b) TMS and OEMS results for LiCoO2[21]. (c) Proposed Mechanism for the Chemical Oxidation of Ethylene Carbonate[8] |
图3 常用(a)含氟溶剂;(b)腈类溶剂;(c)砜类溶剂;(d)离子液体的分子结构Fig. 3 The molecular structures of some (a) fluorinated solvents; (b) nitrile-based solvents; (c) sulfone-based solvents; (d) ionic-liquid solvents |
图4 (a) 使用不同电解液的Li/LCO电池在4.5和4.6 V恒压下的漏电流[23];(b) TFP电解液在电池中的作用示意图[42];(c) 砜类和碳酸酯分子和溶剂化分子的氧化稳定性对比[6];(d) PP13TFSI离子液体电解液循环稳定性[43]Fig. 4 Schematic diagram of (a) Leakage currents during 4.5 and 4.6 V constant voltage floating tests of Li/LCO cells using different electrolytes [23], (b) the effect of TFP electrolyte in the cells[42]. (c) Comparison of oxidation stability between sulfone and carbonate molecules and solvated molecules [6]. (d) Cycle performance of ionic liquid electrolyte with PP13TFS[43] |
图5 (a)LiPF6和LiFSI对Al集流体作用示意图;(b)3~4.6 V LiFSI电解液循环100圈后NCM811颗粒和Al集流体的SEM图[63];(c)传统电解液和LHCE对Al腐蚀的影响示意图[61]Fig. 5 (a) Schematic diagram of the effect of LiPF6 and LiFSI on Al current collector [65]; (b) SEM of NCM811 particles and Al collector after 100 cycles of 3~4.6V with LiFSI electrolyte [63]; (c) Schematic diagram of the influence of traditional electrolytes and LHCE on Al corrosion[61] |
表1 新型电解液体系电化学性能Table 1 Electrochemical performance of novel electrolyte |
| Strategy | Electrolyte | Electrochemical system | Cut-off Voltage/V | Capacity Retention | Ref |
|---|---|---|---|---|---|
| Fluorinated | 2 mol/L LiPF6- DMC/FEC/FEMC | LiCoO2/Graphite | 4.5 | 74.2%(0.5 C,270 cycles) | 23 |
| 2 mol/L LiPF6- DMC/FEC/FEMC | LiNi0.5Mn1.5O4/Li | 5 | 89.9%(0.5 C,300 cycles) | 23 | |
| 1 mol/L LiPF6-FEC/FEMC/HFTFE | LiNi0.92Co0.04Mn0.04O2/Li | 5 | 68%(1 C, 500 cycles) | 24 | |
| 1.2 mol/L LiPF6-FEC/DFEC/FEMC | NCM811/Li | 4.4 | 90.8%(0.5 C, 200 cycles) | 25 | |
| 1 mol/L LiPF6-FEC/MTFP | NCM811/Li | 4.5 | 80%(0.5 C, 250 cycles) | 29 | |
| 1 mol/L LiTFSI- MDFA/MDFSA/TTE | NCM811/Graphite | 4.5 | 80.1%(0.5 C, 400 cycles) | 30 | |
| Nitrile | 1 mol/L LiDFOB-ADN/DMC | NCM111/graphite | 4.5 | 77%(0.5 C, 40 cycles) | 41 |
| 10 mol/L LiFSI-AN+VC | NCM111/Li | 4.5 | 85%(3.6 mAcm-2, 40 cycles) | 44 | |
| 1 mol/L LiTFSI-FEC/PN | NCM622/Li | 4.5 | 75.3%(1 C, 300 cycles) | 42 | |
| LiFSI-SN/AN | LiNi0.5Mn1.5O4/Li | 4.9 | 90.4%(0.1 C,100 cycles) | 46 | |
| NCM811/Li | 4.4 | 73%(0.1C, 200 cycles) | 46 | ||
| Sulfone | 1.2 mol/L LiPF6-FEC/TFPMS | NCM622/graphite | 4.5 | 71%(0.5 C, 400 cycles) | 49 |
| 1 mol/L LiPF6-EMS/DMC | LiNi0.5Mn1.5O4/Li | 4.9 | 97%(0.2 C, 100 cycles) | 50 | |
| 1 mol/L LiFSI-SL/FEC/HFE | NCM811/Li | 4.7 | 84.95%(0.5 C, 150 cycles) | 51 | |
| 1 mol/L LiTFSI-TMS/FEC | NCM811/Li | 4.4 | 86%(0.5 C, 500 cycles) | 48 | |
| Ionic-Liquid | 2.4 mol/L LiTFSI/PMP-FSI | LNMO/graphite | 5 | 85%(0.5 C, 200 cycles) | 56 |
| 1 mol/L LiFSI+0.3 mol/L LiNO3-PP13TFSI/DME | NCM811/Li | 4.3 | ~72%(5 C, 600 cycles) | 43 | |
| LHCE | LiFSI-DME/FEC/PFPN | NCM811/ graphite | 4.6 | 89.8%(0.33 C, 300 cycle) | 65 |
| 2.0 mol/L LiFSI/DME-BTFMD | NCM811/Li | 4.4 | 87%(1 C, 250 cycle) | 69 | |
| 1 mol/L LiFSI/TMS-TTE | LNMO/Li | 4.9 | ~93%(1 C, 100 cycles) | 70 | |
| Dual Salt | 1 mol/L LiPF6+0.1 mol/L LiFSI+0.1 mol/L LiBF4 | Li1.2Mn0.54Ni0.13Co0.13O2/Li | 4.8 | 80.4%(1 C, 400 cycle) | 73 |
| 0.8 mol/L LiFSI+0.1 mol/L LiTFSI+ 0.6 mol/L LiPF6-EMC | NCM811/graphite | 4.5 | 82.1%(1 C, 200 cycle) | 74 |
图9 (a)空白电解质和(b)含HBCHHI的电解质中SEI和CEI的保护机制示意图[102];(c)ADMF添加剂作用机理[103];(d)在0.5C、3.0~4.55 V含或不含ADMF的LCO/MCMB全电池的循环性能和库仑效率[103]Fig. 9 Schematic illustration of the protection mechanism of the SEI and CEI in (a) blank electrolyte and (b) HBCHHI-contained electrolyte [102]. (c) Schematic diagram of the mechanism of ADMF additive [103]. (d) Cycling performance and Coulombic efficiency versus cycle number of LCO/MCMB full-cells with or without ADMF at 0.5 C at 3.0~4.55 V [103]. |
表2 高压电解液成膜添加剂电化学性能Table 2 Electrochemical performance of typical additive in high-voltage electrolyte |
| Additive | Electrolyte | Electrochemical system | Cut-off Voltage/V | Capacity Retention | Ref |
|---|---|---|---|---|---|
| Phosphorous additive | 1 mol/L LiPF6-EC/DEC+0.5wt%EMPA | NCM523/Gr | 4.5 | 63% (0.5 C, 400 cycles) | 76 |
| NCM111/Gr | 4.5 | 200 cycles) | |||
| 4.6 | 300 cycles) | ||||
| 1 mol/L LiPF6-EC/DEC+1%TPP | NCM523/AG | 4.6 | 88.2%) | 77 | |
| 1.2 mol/L LiPF6-EC/EMC+1%TTFP | NCM523/graphite | 4.6 | 99.7% (C/3, 50 cycles) | 75 | |
| 1.3 mol/L LiPF6-EC/EMC/DMC+1%LiDFBP | Li1.17Ni0.17Mn0.5Co0.17O2/Li | 4.6 | 90% (0.5 C, 100 cycles) | 80 | |
| Boronated additive | 1 mol/L LiPF6-EC/EMC+0.1%TEAB | NCM811/Li | 4.3 | 63.2% (100 cycles) | 81 |
| 1.3 mol/L LiPF6-EC/EMC/DMC+1%LiDFOB | Li1.17Ni0.17Mn0.5Co0.17O2/graphite | 4.7 | 82.7% (0.5 C, 100 cycles) | 82 | |
| 1 mol/L LiPF6-EC/DMC+2%LiBOB | Li1.18Ni0.18Mn0.55Co0.09O2/graphite | 4.7 | 89.5% (0.2 C, 200 cycles) | 85 | |
| 1.0 mol/L LiPF6-EC/DMC+1%LiBOB | LiNi0.83Co0.11Mn0.05B0.01O2/Li | 4.6 | 73.1% (1 C, 200 cycles) | 88 | |
| LiDFOB+LiPO2F2+LiPF6-EMC/DMC | NCM811/Li | 4.7 | 80% (1 C, 500 cycles) | 89 | |
| 1 mol/L LiPF6-EC/EMC+5%PFPBA | NCM622/Li | 4.6 | 91.2% (1 C, 400 cycles) | 90 | |
| S-containing additive | 1 mol/L LiPF6-EC/EMC +1%PS | NCM811/Li | 4.45 | 80% (0.5 C, 200 cycle) | 93 |
| 1 mol/L LiPF6-EC/EMC +1%P-DTD | NCM811/graphite | 4.4 | >90% (1 C, 500 cycles) | 96 | |
| 1 mol/L LiPF6-EC/EMC +1%MMDS | NCM523/graphite | 4.5 | 92.78% (1 C, 800 cycle) | 97 | |
| 1 mol/L LiPF6-EC/EMC +1%MMDS | NCM523/graphite | 4.6 | 800 cycle) | ||
| 1 mol/L LiPF6-FEC/EMC/DMC +1% NTSA | LiCoO2/ω-LVO | 4.35 | 94.5% (200 cycle) | 99 | |
| 1 mol/L LiPF6-EC/EMC/DMC +FEC+EVS | Li1.17Ni0.265Co0.047Mn0.517O2/Li | 4.8 | 97% (1 C, 300 cycles) | 100 | |
| 1 mol/L LiPF6-EC/DMC +0.5%BFS | LiCoO2/Li | 4.6 | 88% (1 C, 300 cycles) | 101 | |
| Other additive | 1 mol/L LiPF6-EC/EMC/DEC +5m mol/L HBCHHI | NCM811/Li | 4.7 | 63.6% (5 C, 500 cycles) | 102 |
| 1 mol/L LiPF6-EC/DMC/DEC +1%SN+0.1%CHB | LiCoO2/Li | 4.6 | 77.4% (1 C, 500 cycles) | 104 | |
| NCM811/Li | 4.7 | 600 cycles) | |||
| 1 mol/L LiPF6-EC/DMC +1%ADMF | LiCoO2/MCMB | 4.55 | 87% (0.5 C, 750 cycles) | 103 | |
| NCM811/MCMB | 4.4 | 750 cycles) | |||
| 1 mol/L LiPF6-FEC/EMC +1%TPFPB+3wt%LiNO3 | LiCoO2/Li | 4.6 | 89.8% (0.2 C, 160 cycles) | 105 |
图10 (a)含有Si—O和Si—N官能团的化合物;(b)含有—NCO官能团的化合物;(c)含有酸酐官能团化合物的结构式Fig. 10 The molecular structures of (a) Compounds containing Si—O and Si—N functional groups. (b) Compounds containing —NCO functional groups. (c) Compounds containing acid anhydride functional groups |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
/
| 〈 |
|
〉 |