

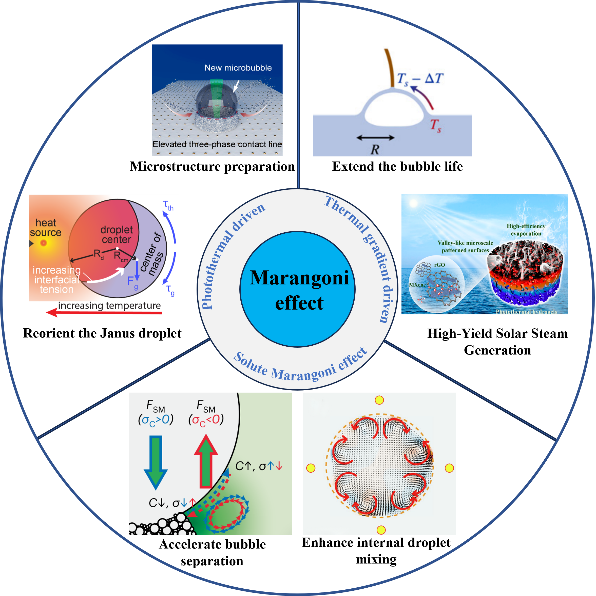
Microbubble/Droplet Manipulation Based on Marangoni Effect
Received date: 2024-02-22
Revised date: 2024-07-21
Online published: 2024-09-15
Supported by
National Natural Science Foundation of China(11972150)
Microbubbles and microdroplets, when exposed to a uniform temperature gradient/solute concentration gradient, will undergo thermal capillary migration/solute migration, leading to the emergence of the Marangoni effect at the gas-liquid interface. This effect plays a crucial role in manipulating microbubbles or microdroplets, offering valuable applications in various fields including biology, chemistry, medicine, materials science, and micromanufacturing. In this review, provided are an overview of recent advancements about the Marangoni effect of microbubbles/droplets under different driving modes, and demonstrate the driving principle and characteristics of photothermal Marangoni effect, thermal gradient-driven Marangoni effect and solute Marangoni effect. We focus on the dynamic changes of microdroplets induced by photothermal Marangoni effect, the movement principles of droplets on diverse hydrophobic surfaces, the manipulation processes of bubble movement and bubble separation under laser irradiation, and the typical instances of bubble/droplet separation, droplet evaporation and mixing achieved through thermal gradient-driven Marangoni effect and solute Marangoni effect. Furthermore, recent applications of the Marangoni effect in microbubble/droplet manipulation are highlighted and the promising future prospects for further development and utilization of this phenomenon are discussed.
Contents
1 Introduction
2 Driving principle of the Marangoni effect
3 Temperature driven Marangoni effect
3.1 Photothermal Marangoni effect of microdroplets/ bubbles
3.2 Thermal gradient Marangoni effect of microdroplets/ bubbles
4 Microdroplet/bubble solute Marangoni effect
5 Application based on microdroplet/bubble Marangoni effect
5.1 Preparation of surface microstructure
5.2 Bubble-pen lithography
5.3 Multiphase droplet drive
5.4 Droplet motor
5.5 Emulsion energy supply
6 Conclusion and prospect
Zhenlin Wei , Hongfei Wang , Yaliang Chen , Junbo Xing , Dayong Li . Microbubble/Droplet Manipulation Based on Marangoni Effect[J]. Progress in Chemistry, 2024 , 36(10) : 1541 -1558 . DOI: 10.7536/PC240215
图2 不同驱动方式下Marangoni效应实现对微气泡/液滴操控的相关研究:(a)驱动疏水表面上的液滴[33];(b)控制液滴定向旋转[20];(c)控制气泡周围粒子流动[34];(d)诱发液滴内部涡流[35];(e)增强沟槽内液体流速[13];(f)延长受热气泡寿命[36]Fig. 2 Research on microbubble/droplet manipulation by Marangoni effect with different driving modes (a) drive droplet on the hydrophobic surface [33]; (b) control droplet rotating directionally [20]; (c) control particle flow around the bubble [34]; (d) induce vortex flow inside a droplet [35]; (e) enhance liquid flow rate in groove structure [13]; (f) extend the life of a heated bubble [36] |
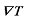 ) produced after the droplet absorbs light energy causes a surface tension gradient (∇γ), thus inducing an internal photothermal Marangoni flow within the droplet, which flows in the opposite direction to the temperature increase. This circulating flow inside the droplet promotes mass transfer within it and alters the shape of the droplet's surface. With continuous laser irradiation, the presence of the surface tension gradient continuously affects the dynamic behavior of the droplet, causing directional movement. In applications related to bubble control using the Marangoni effect, the photothermal Marangoni effect leads to an increase in the temperature of the liquid around the bubble, thereby changing the surface tension and buoyancy of the bubble. This changes the speed at which the bubble rises or falls, ultimately affecting the stability and morphology of the bubble, triggering separation or oscillation. When the bubble oscillates in the liquid, the upward and downward Marangoni forces it experiences are
) produced after the droplet absorbs light energy causes a surface tension gradient (∇γ), thus inducing an internal photothermal Marangoni flow within the droplet, which flows in the opposite direction to the temperature increase. This circulating flow inside the droplet promotes mass transfer within it and alters the shape of the droplet's surface. With continuous laser irradiation, the presence of the surface tension gradient continuously affects the dynamic behavior of the droplet, causing directional movement. In applications related to bubble control using the Marangoni effect, the photothermal Marangoni effect leads to an increase in the temperature of the liquid around the bubble, thereby changing the surface tension and buoyancy of the bubble. This changes the speed at which the bubble rises or falls, ultimately affecting the stability and morphology of the bubble, triggering separation or oscillation. When the bubble oscillates in the liquid, the upward and downward Marangoni forces it experiences are 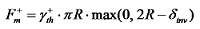 ,
, 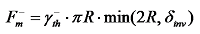 respectively. Here,
respectively. Here, 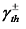 represents the surface tension gradient, R represents the radius of the bubble, and
represents the surface tension gradient, R represents the radius of the bubble, and 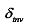 represents the thickness of the inversion layer (a longitudinal temperature distribution that first increases then decreases due to the rapid cooling effect of the wall). The Marangoni force, gravity, and buoyancy collectively act on the bubble, influencing its entire oscillation process[54]. Figure 3(b) depicts the principle diagram of the thermally driven Marangoni effect. In a heated silicon oil bath, when air (at room temperature) is introduced, upon reaching the surface of the silicon oil bath, a temperature difference between the cooler top region of the bubble and the warmer bottom region of the oil bath generates an upward Marangoni effect (convection) around the bubble, making the direction of material transport opposite to gravity, which balances the forces acting on the bubble, prolonging its existence. The temperature difference-induced thermal Marangoni convection between the top and bottom of the bubble affects the overall balance of the bubble, with the development direction of the equilibrium depending on the direction of the temperature gradient. Generally, the intensity of the temperature-driven Marangoni effect is characterized by the formula Ma=
represents the thickness of the inversion layer (a longitudinal temperature distribution that first increases then decreases due to the rapid cooling effect of the wall). The Marangoni force, gravity, and buoyancy collectively act on the bubble, influencing its entire oscillation process[54]. Figure 3(b) depicts the principle diagram of the thermally driven Marangoni effect. In a heated silicon oil bath, when air (at room temperature) is introduced, upon reaching the surface of the silicon oil bath, a temperature difference between the cooler top region of the bubble and the warmer bottom region of the oil bath generates an upward Marangoni effect (convection) around the bubble, making the direction of material transport opposite to gravity, which balances the forces acting on the bubble, prolonging its existence. The temperature difference-induced thermal Marangoni convection between the top and bottom of the bubble affects the overall balance of the bubble, with the development direction of the equilibrium depending on the direction of the temperature gradient. Generally, the intensity of the temperature-driven Marangoni effect is characterized by the formula Ma= 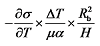 , where Ma represents the Marangoni number, σ denotes surface tension (N/m); T denotes temperature (K); ΔT denotes the maximum temperature difference in the system (K); μ denotes the dynamic viscosity of the solution (N·s/m2), and α represents the thermal diffusivity of the solution (m2/s), Rb represents the bubble radius (m), and H represents the height of the liquid layer (m)[23]; the larger the Marangoni number, the stronger the Marangoni convection. Figure 3(c) illustrates the principle of the generation of the solute Marangoni effect. The blue circle on the left represents a stationary water droplet, the gray tubular shape on the right is a capillary tube (containing ethanol), and the orange area in the middle represents ethanol diffused from the capillary tube into the air. R is the radius of the droplet, and d is the distance from the droplet to the capillary tube. Since the surface tension of ethanol is lower than that of water, and the concentration of ethanol is higher in the central part of the right side of the droplet closer to the capillary tube, a flow from the center to the periphery (indicated by red arrows) occurs on the right side of the droplet. This convective phenomenon caused by the dissolution or chemical reaction of a certain component in the system is known as the solute Marangoni effect[22,55]. When a liquid film is locally thinned due to temperature or concentration disturbances, it forms a solute Marangoni flow under the action of the surface tension gradient, allowing the liquid to flow back along the optimal path to the thin liquid surface. Because the region with higher surface tension exerts a greater pull on the surrounding liquid, the liquid flows away from areas of lower surface tension. It can be seen that the solute Marangoni effect is a type of liquid flow phenomenon caused by uneven surface tension. Although the external manifestations of the three driving methods differ, fundamentally, all three arise from the presence of a surface tension gradient, meaning the liquid flows from areas of lower surface tension to areas of higher surface tension[56].
, where Ma represents the Marangoni number, σ denotes surface tension (N/m); T denotes temperature (K); ΔT denotes the maximum temperature difference in the system (K); μ denotes the dynamic viscosity of the solution (N·s/m2), and α represents the thermal diffusivity of the solution (m2/s), Rb represents the bubble radius (m), and H represents the height of the liquid layer (m)[23]; the larger the Marangoni number, the stronger the Marangoni convection. Figure 3(c) illustrates the principle of the generation of the solute Marangoni effect. The blue circle on the left represents a stationary water droplet, the gray tubular shape on the right is a capillary tube (containing ethanol), and the orange area in the middle represents ethanol diffused from the capillary tube into the air. R is the radius of the droplet, and d is the distance from the droplet to the capillary tube. Since the surface tension of ethanol is lower than that of water, and the concentration of ethanol is higher in the central part of the right side of the droplet closer to the capillary tube, a flow from the center to the periphery (indicated by red arrows) occurs on the right side of the droplet. This convective phenomenon caused by the dissolution or chemical reaction of a certain component in the system is known as the solute Marangoni effect[22,55]. When a liquid film is locally thinned due to temperature or concentration disturbances, it forms a solute Marangoni flow under the action of the surface tension gradient, allowing the liquid to flow back along the optimal path to the thin liquid surface. Because the region with higher surface tension exerts a greater pull on the surrounding liquid, the liquid flows away from areas of lower surface tension. It can be seen that the solute Marangoni effect is a type of liquid flow phenomenon caused by uneven surface tension. Although the external manifestations of the three driving methods differ, fundamentally, all three arise from the presence of a surface tension gradient, meaning the liquid flows from areas of lower surface tension to areas of higher surface tension[56].图3 3种不同Marangoni效应驱动方式的原理图:(a)液滴的激光照射区域与液滴边界区域之间的温度梯度诱导光热Marangoni效应原理图;(b)在加热液体表面,从气泡底部受热区域流向气泡顶部较冷区域的热梯度驱动的Marangoni效应原理图,比例尺:3 mm;(c)由挥发性液体(如乙醇)在固着液滴表面的不均匀分布诱导的溶质Marangoni效应原理图,R为液滴半径,d为液滴右边界与毛细管(内含挥发性液体)左端的距离Fig. 3 Schematic diagrams of three different driving modes of Marangoni effect: Schematic diagrams of (a) Photothermal Marangoni effect induced by temperature gradient between laser irradiation area and droplet boundary area; (b) Marangoni effect driven by thermal gradient from the heated area at the bottom of a bubble to the cold area on the top of the gas bubble; (c) Solute Marangoni effect induced by uneven distribution of volatile liquid (such as ethanol) on the surface of a sessile droplet, where R is droplet radius, and d represents the distance between the right boundary of droplet and the left end of capillary (containing volatile liquid) |
图4 近场红外激光对聚吡咯液滴的控制过程(a)纳米颗粒质量分数为1%,体积为10 µL的聚吡咯(PPy)液滴在润滑表面(Lubricated Surfaces)上的运动;(b)近红外辐射下去离子水液滴(10 µL)在润滑表面(LuS)上的运动;(c)近红外辐射驱动下润滑表面(LuS)上PPy液滴运动的原理图[33]Fig. 4 Manipulation process of a polypyrrole droplet by near-infrared (NIR) laser: Motion of (a) a polypyrrole droplet and (b) a deionized water droplet on lubricated surface; (c) schematic diagram of polypyrrole droplet motion on lubricated surface driven by near-infrared radiation[33] |
图5 Marangoni流和曲率诱导的涡旋(a) 4×放大倍数,(b) 10×放大倍数,(c) 20×放大倍数,(d) 40×放大倍数时,碳纳米管在涡旋区的聚集情况。白色圆圈表示激光聚焦的位置(e)焦平面上激光光斑处的温度场模拟(f)焦平面上激光光斑处的速度场模拟[73]Fig. 5 Vortex induced by Marangoni flow and curvature: The aggregation of carbon nanotubes in the vortex region (a) 4× magnification; (b) 10× magnification; (c) 20× magnification and (d) 40× magnification. The white circle indicates where the laser is focused; (e) simulation of temperature field at the laser spot on the focal plane and (f) simulation of velocity field at the laser spot on the focal plane [73] |
图6 光热Marangoni效应捕获微气泡(a)含有溶解染料的液体溶液吸收激光诱导表面Marangoni流动的可视化和(b)仿真得到的粒子的轨迹与速度分布图像,红色表示速度快,蓝色表示速度慢;(c)气泡被Marangoni效应捕获的现象示例,箭头表示气泡移动方向[88]Fig. 6 Microbubbles captured by photothermal Marangoni effect:(a) visualization of laser-induced surface Marangoni flow absorbed by a liquid solution containing dissolved dyes; (b) simulated particle trajectory and velocity distribution images, red colour indicates fast velocity and blue colour indicates slow velocity; (c) an example of a gas bubble captured by Marangoni effect, the arrows indicates the direction of bubble movement [88] |
图7 未脱气水和脱气水中的水蒸气气泡周围的Marangoni流动:(a)未脱气水中气泡和(b)脱气水中水蒸气微泡周围的流体流动。小黑点是聚苯乙烯(PS)球体,用来可视化流体运动。PS球的轨迹表示与气泡周围的旋转流相比,蒸气泡周围的旋转流更快而且更大。(c)、(d)分别给出了(a)、(b)中的流动方向的示意图[90]Fig. 7 Marangoni Flow around a vapor bubble in degassed and undegassed water: Fluid flow around (a) air bubble in undegassed water and (b) vapor bubble in degassed water. The small black dots are polystyrene (PS) spheres that are used to visualize fluid movement. The trajectory of PS balls indicates that the rotating flow around the vapor bubble is faster and larger as compared with that around the air bubble; (c) and (d) gives a sketch of the flow directions in (a) and (b) respectively[90] |
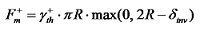 ) gradually decreases, and the downward Marangoni force (
) gradually decreases, and the downward Marangoni force ( 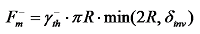 ) starts to increase significantly (where
) starts to increase significantly (where 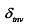 represents the thickness of the TIL, and
represents the thickness of the TIL, and 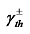 represents the surface tension gradient), causing the bubble to move downwards. With changes in the position and motion state of the bubble (Figure 8b, c), the direction of the thermal Marangoni force switches from upward to downward, leading to the bouncing behavior of the bubble. The flow field simulation in Figure 8(d) shows the direction change of the Marangoni flow, inducing the reverse movement of the bubble (t=(t 0+15) ms).
represents the surface tension gradient), causing the bubble to move downwards. With changes in the position and motion state of the bubble (Figure 8b, c), the direction of the thermal Marangoni force switches from upward to downward, leading to the bouncing behavior of the bubble. The flow field simulation in Figure 8(d) shows the direction change of the Marangoni flow, inducing the reverse movement of the bubble (t=(t 0+15) ms).图8 自发弹跳气泡(a)实验装置示意图以及在近红外激光(波长为980 nm,P= 15 W)照射水面后,水中产生的气泡弹跳过程的图像(红色框中呈现了气泡弹跳行为的两个典型周期,t=0 s表示气泡可见之前的时刻;Ht和Hc分别表示气泡的顶部和中心到水面的距离);(b)表示气泡在液体中运动时的浮力和热Marangoni力的变化过程(c)表示靠近壁面运动时气泡受到的浮力和热Marangoni力的变化关系;(d) 弹跳气泡速度场的数值模拟图像;(e)气泡(ii和iii)与弹跳气泡(i)(移动速度为5 mm/s)的聚结过程。(f) 从侧面和顶面观察了正己烷液滴对弹跳气泡的包裹作用,形成了核壳结构的气泡-液滴复合物。(g) 激光诱导下的弹跳气泡在扫过水面时,收集到直径为80 μm的PS微珠,比例尺:200 μm[93]Fig. 8 Spontaneous bouncing bubbles:(a)Schematic diagram of the experimental setup and images showing the bubble bouncing process generated in water after the near-infrared laser (wave length 980 nm, P= 15 W) irradiating the water (the red box presents two typical cycles of the bubble bouncing behavior, t=0 s represents the moment before the bubble is visible; Ht and Hc indicate the distance from the top and center of the bubble to the water surface, respectively). The change in buoyancy and thermal Marangoni force for (b) a bubble moving in the liquid and for (c) the bubble moving near the wall; (d) The numerical simulation results showing the velocity field of a bouncing bubble; (e) The coalescence process of bubbles; (f) Side and top views about the core shell structure of bubble droplet composite material obtained by encapsulating dancing bubbles with dye hexane droplets; (g) Particle (PS beads with diameter of 80 μm) collection onto the surface of the bouncing bubble as its sweeping through the water[93] |
图9 激光脉冲控制气泡弹跳高度及频率:(a)光热Marangoni效应主导的气泡向下运动的机理图,(b)在激光照射功率为57 mW时,使用COMSOL模拟的气泡开始向下移动时(ton = 2277 μs)的温度场,(c)温度和温度梯度作为在垂直方向的变化函数,(d,I)等离子体气泡向下移动过程的PIV测量图像。激光照射后,光热诱导的Marangoni流变强,推动气泡向下运动,直到气泡到达衬底,(d,II)气泡向下运动过程中温度场(图左)和流场(图右)的数值模拟,(e)在水中拍摄的等离子体泡一个周期内的光学图像,比例尺:10 μm[94]Fig. 9 Height and frequency of a bouncing bubble controlled by laser pulse:(a) Mechanism diagram of downward movement of a gas bubble dominated by photothermal Marangoni effect; (b) Temperature field of a bubble starting to move downward simulated by COMSOL under laser irradiation with power of 57 mW; (c) temperature and temperature gradient as functions of vertical direction; (d, I) PIV measurement image showing downward movement of a plasma bubble; (d, II) Numerical simulation of temperature field (left) and flow field (right) during the downward movement of the bubble; (e) Optical image of a bouncing plasmonic bubble in water during one cycle (I, scale:10 μm) with the on/off control of laser shown in (II) [94] |
图10 溶质Marangoni流的时间演变过程:(a)乙醇蒸气源数量为1个且位于液滴右侧,橙色虚线圆圈表示液滴的接触线。(b)存在1个丙酮蒸气源时溶质Marangoni效应的混合实验;(c)存在2个丙酮蒸气源时溶质Marangoni效应的混合实验;(d)没有任何蒸气源时液滴内部的纯扩散[35]Fig. 10 Temporal evolution of the solutal Marangoni flow:(a) The number of ethanol vapor sources is 1 and is located on the right side of the droplet, the orange-dashed circle represents the contact line of the droplet. Mixing experiments with solutal Marangoni effects; (b) one point of acetone vapor source; (c) two points of acetone vapor sources; (d) pure diffusion inside the droplet in the absence of any vapor source [35] |
图12 3D表面微结构制造:(a)由于等离子体效应,气泡周围的热Marangoni流将PS微粒运输到三相接触线。(b)关闭激光后,气泡从基板上分离并上升,在基板上留下微结构。(c)等离子体气泡的反复成核并分离导致PS微粒在底部积累,(d)等离子体气泡重复成核,最终形成了三维微柱,(e)表面微结构产生后等离子体气泡周围的Marangoni对流仿真图像,(f)(f,Ⅰ)在激光功率为302 mW,在占空比为ton(单个周期中激光打开的时间)=200 ms,toff(单个周期中激光关闭的时间)=200 ms,N(气泡重复成核的次数)=100,c(PS浓度)=150 μg/mL时,5倍物镜观测下三维微结构的等距扫描电子显微镜图像。(f,II)、(f,III)和(f,IV)分别是(f,Ⅰ)中所示的1、2、3三个选定区域的扫描电子显微镜图像,(g)在没有表面微结构和有表面微结构(h)的情况下通过跟踪流体中PS微粒的运动构建的Marangoni对流流线[21]Fig. 12 3D fabrication of surface microstructure:(a) The transport of PS particles to the three-phase contact line due to the plasma effect in the presence of a hot Marangoni flow around a bubble; (b) The bubble detaches from the substrate once the laser is turned off, leaving behind a microstructure on the substrate; (c) The repeated nucleation and separation of plasma bubbles result in the accumulation of PS particles at the bottom; (d) The repeated nucleation of plasma bubbles eventually leads to the formation of a three-dimensional microcolumn; (e) The image shows a simulation of Marangoni convection around a plasma bubble after the generation of surface microstructure; (f, I) An isometric view of SEM image showing an obtained microstructure with a 5× objective lens, the experimental parameters used were Pl = 302 mW, ton = 200 ms, toff = 200 ms, N = 100, and c = 150 μg/mL, (f, II), (f, III), and (f, IV) are enlarged SEM images of three selected areas shown in (f, I); (g) The Marangoni convection streamline constructed by tracking the motion of PS particles in the fluid without surface microstructure; (h) Conversely, with the surface microstructure present, the Marangoni convection streamline is affected [21] |
图13 等离子体增强光热效应气泡笔光刻:(a)单个微泡捕获粒子机制的示意图(截面图)。蓝色球体表示去离子水中的悬浮粒子。由于摩擦力的作用,粒子沿着Marangoni对流流动。插图显示了粒子被微气泡捕获时的力分布(红色虚线表示)。PB和PL分别表示气泡中的压力和液体中的压力,它们产生将粒子向外推的净力FP。表面张力FS产生拖曳力FD。(b)在AuNIs基板上连续写入540 nm PS小球直线图案的时间分辨过程,比例尺:50 μm。(c) 540 nmPS小球的“SP”图案的暗场光学图像,比例尺:10 μm。(d) 60 nm PS小球的4×4阵列三维空心结构的暗场光学图像,比例尺:10 μm[125]Fig. 13 Plasma-enhanced photothermal bubble pen lithography:(a) Schematic diagram of the particle trapping mechanism of a single microbubble. The blue spheres represent suspended particles in deionized water. These particles flow convection along the Marangoni due to friction. As shown in the inset, a particle is captured by the microbubble indicated by the dotted red line. The inner pressure of the bubble (PB) and the pressure in the liquid (PL) produce a net force (FP) that pushes the particles outward. Additionally, the surface tension (FS) introduces a drag force (FD), as illustrated; (b) Time-resolved process of continuous writing in straight line pattern performed on the AuNIs substrate (with 540 nm PS bead, scale: 50 μm); (c) Dark field optical image revealing the formation of the "SP" letter pattern (540 nm PS beads, scale :10 μm); (d) Dark field optical image demonstrating a 4×4 array of 3D hollow structures (60 nm PS beads, scale:10 μm) [125] |
图14 乳化液滴朝向激光点重新定位:(a)庚烷和全氟己烷形成的乳化液滴旋转朝向热源重新定位过程,比例尺为50 µm。(b)仅受重力影响时,由较轻碳氢化合物(粉红色)和较重氟碳化合物(灰色)形成的乳化液滴的俯视图;(c)热源经过时乳化液滴的俯视图示意图;(d)同一液滴的侧视图示意图,γH、γF和γFH分别为碳氢化合物、氟碳化合物、两种化合物接触面的界面张力;(e) 液滴内部毛细表面形成界面张力梯度,使液滴在平衡热Marangoni力扭矩τth和重力扭矩τg的情况下呈稳态倾斜。Rd:液滴半径,Rcm:重心到质心的距离,Fg:重力[20]Fig. 14 Repositioning of emulsion droplets toward the laser point:(a) Emulsion droplets consisting of perfluorohexane and heptane were observed to rotate towards the heat source (scale: 50 µm); (b) and (c) present top-views of the emulsion droplets formed from lighter hydrocarbons (pink) and heavier fluorocarbons (grey), (b) under the influence of gravity alone and (c) when a heat source passed by; (d) presents a side view of the same droplet, where γH, γF and γHF are the relevant interfacial tension; (e) An interfacial tension gradient forms at the internal capillary surface of the droplet in a thermal field, and this gradient results in a steady-state tilt with the condition that the thermal Marangoni force torque τth and the gravitational torque τg are balanced. Here, Rd represents the droplet radius, Rcm denotes the distance from the center of gravity to the center of mass, and Fg represents gravity[20] |
图16 油/水界面Marangoni效应诱发湍流:(a)Marangoni效应(湍流)产生前油水界面的状况;(b)Marangoni效应(湍流)产生时油水界面的状况;(c)Marangoni效应引发湍流的示意图;(d)Marangoni效应的传质[133]Fig. 16 Turbulence induced by Marangoni effect at the oil/water interface:(a) The oil-water interface at the beginning of the Marangoni effect (turbulence); (b) The oil-water interface in the process of the Marangoni effect (turbulence); (c) Schematic diagram of turbulenc caused by Marangoni effect; (d) Mass transfer of the Marangoni effect [133] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
(张艳, 郑连存, 张欣欣. 物理学报, 2009, 58(08): 5501.)
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
(陈俊, 沈超群, 王贺, 张程宾. 物理学报, 2019, 68(7): 183.)
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
(胡学铮, 陈烨璞, 倪邦庆, 虞学俊. 物理化学学报, 1998, 14(2): 136.)
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
(沙勇, 成弘, 余国琮. 化学进展, 2003, 15(01): 9.)
|
| [52] |
(唐修行, 陈泓樾, 王婧婧, 王志军, 臧渡洋. 物理学报, 2023, 72(19): 194.)
|
| [53] |
(秦威广, 王进, 纪文杰, 赵文景, 陈聪, 蓝鼎, 王育人. 物理学报, 2022, 71(06): 359.)
|
| [54] |
(胡学铮. 物理化学学报, 1997, 13(10): 873.)
|
| [55] |
(吴凯. 物理化学学报, 2019, 35(11): 1183.)
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
(李震东, 赵建福, 秦文韬. 工程热物理学报, 2010, 31(6): 979.)
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
(邓环环, 张敏, 刘欢. 中国科学: 化学, 2023, 53(7): 1172.)
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
(林圣享, 朱宝杰, 易义武, 张莹, 刘佩尧, 马明. 计算力学学报, 2019, 36(1): 52.)
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
(王子昂, 郭航, 荣欣, 董桂芳. 物理化学学报, 2019, 35(11): 1259.)
|
| [104] |
(韩国彬, 吴金添, 徐晓明. 物理化学学报, 2000, 16(6): 507.)
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
(黄彦如, 黄睿雯, 马雪, 李真珍, 滕宏辉. 力学学报, 2023, 1.)
|
| [112] |
(韩国彬. 物理化学学报, 1998, 14(8): 709.)
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
(张立娟, 郑晋, 文博, 胡钧. 中国科学: 化学, 2024, 54(1): 85.)
|
| [136] |
|
/
| 〈 |
|
〉 |