

Research Progress of Nanofiber Composite Hydrogels
Received date: 2024-03-04
Revised date: 2024-03-28
Online published: 2024-04-16
Supported by
Ningxia Key Research and Development Project(2022BEG02006)
Tianjin Key Research and Development Project(20YFZCSN00130)
Central Government of Heilongjiang Province Guides Funds(ZY23CG35)
Ningxia Autonomous Region Flexible Introduction of Science and Technology Innovation team(2021RXTDLX08)
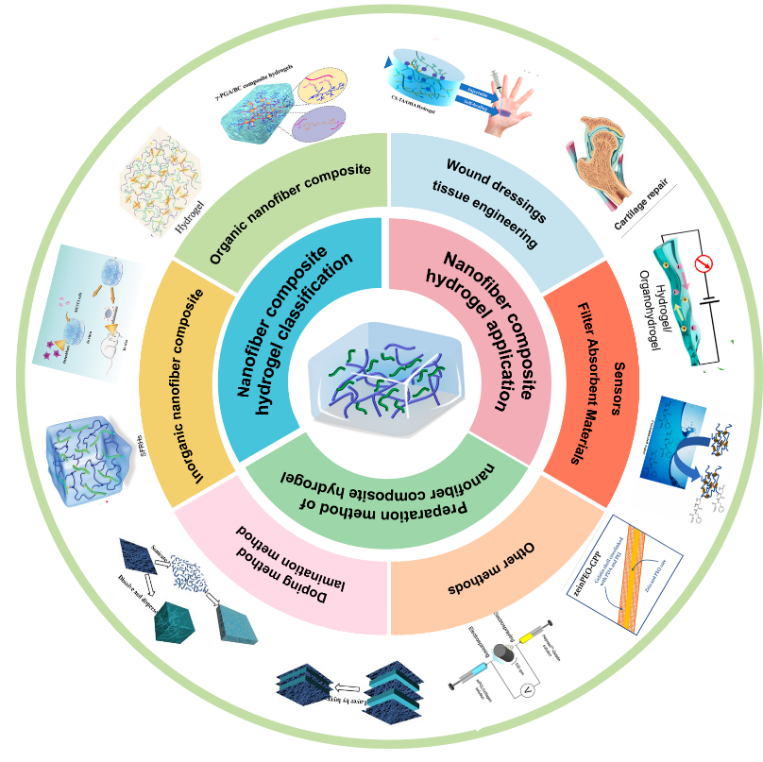
Hydrogels have become one of the most widely researched materials across disciplines due to their excellent softness, wettability, responsiveness and biocompatibility. However, the mechanical properties of hydrogels are poor and cannot meet the use of some special materials. Nanofibers have been used to prepare nanofiber composite hydrogels with nano-size, porous structure and tunable mechanical properties due to their high aspect ratio, uniform fiber morphology and easy functionalization. Nanofiber composite hydrogels have suitable mechanical properties, ductility, adhesion, and the ability to mimic the microstructure of the extracellular matrix (ECM) and the microenvironment of the cell, which makes them widely used in many fields. This paper summarizes the classification of nanofiber composite hydrogels, their preparation methods and their development and application in the fields of multifunctional wound dressings, tissue engineering, sensors, and filter absorption materials future development.
Contents
1 Introduction
2 Nanofiber composite hydrogel classification
2.1 Organic nanofiber composite hydrogel l
2.2 Inorganic nanofiber composite hydrogel
2.3 Organic-inorganic hybrid nanofiber composite hydrogels
3 Preparation method of nanofiber composite hydrogel
3.1 Doping method
3.2 lamination method
3.3 Other methods
4 Nanofiber composite hydrogel application
4.1 Multifunctional wound dressing
4.2 Tissue engineering
4.3 Conductive sensors
4.4 Absorbent filter material for dye and metal ion removal
5 Conclusions and outlook
Key words: nanofiber; hydrogel; composite material; mechanical property
Yvqing Ma , Zheng Li , Guobao Zheng , Songnan Zhang , Jixian Gong , Changsheng Qiao . Research Progress of Nanofiber Composite Hydrogels[J]. Progress in Chemistry, 2024 , 36(10) : 1594 -1606 . DOI: 10.7536/PC240305
表1 纳米纤维复合水凝胶分类及应用Table 1 Nanofiber composite hydrogel classification and applications |
| Nanofiber | Hydrogel material | Features & Applications | Ref |
|---|---|---|---|
| Cellulose nanofibers (CNF) | Gelatine (Gel); Gelatinized methacrylate (GelMA) | 3D bioprinting of biomedical scaffolds. Modulation of gelatin gel properties with potential application in gelatin food additives | 32,33 |
| Bacterial cellulose nanofiber (BC-f) | Polyacrylamide (PAM) composite hydrogels; Poly(γ-glutamic acid) (γ-PGA) hydrogel | High mechanical properties with great potential for wound dressings and biomedical applications. High mechanical strength, on-demand drug release, smart drug delivery patch | 34,35 |
| Polylactic acid (PLA) nanofiber | GelMA Alginate (Alg)-hyaluronic acid (HA) hydrogel (Alg-HA) | Providing a suitable microenvironment for bone tissue engineering repair. Meet the mechanical properties of cartilage tissue, no cytotoxicity, suitable for chondrocyte migration. | 36,37 |
| Polycaprolactone (PCL) nanofiber | Gelatin-based hydrogel; Composite hydrogel | 3D hybrid scaffolds are mimicking natural heart tissue structures. Bone regeneration in bone repair in clinical therapy | 38,39 |
| Aramid nanofiber (ANF) | polyvinyl alcohol (PVA) | Implantable tissue prosthetics, tendon-mimetic hydrogels, flexible Electronic Sensors | 10,40 |
| Silicon dioxide (SiO2) nanofiber | Vinylsilane/Sodium/Alginate/ Polyacrylamide composite hydrogel; Siloxane-derived hydroxy propyl methyl cellulose composite hydrogel | Bionic electronic sensors with high mechanical strength. Injectable materials for minimally invasive cartilage surgery | 25,41 |
| Carbon nanofiber powder (CFP) | Alginate (Alg) based hydrogels | New biomaterial for bone tissue engineering scaffolds, topical drug-delivery carriers | 42,43 |
| Hydroxyapatite nanofiber (HANF) | GelMA | Great potential for application in tissue engineering. | 44 |
| Organic-inorganic hybrid nanofiber | Chitosan (CS) hydrogel; GelMA | Good anti-scaling properties, filtration of dyes in wastewater. Self-assembly of nanofibers within hydrogels for applications in biomimetic scaffolds | 45,46 |
图2 (a)ACH中ANF的增强作用和高度定向的网络[10];(b)APA/CMCS/KGN@PGF/GM制备示意图,具有优异的生物相容性[11];(c)在PCL/Gel纳米纤维上涂覆GelMA凝胶用于组织工程支架[57]Fig. 2 (a) Enhancement of ANF in ACH and highly oriented networks[10]. (b) Schematic diagram of APA/CMCS/KGN@PGF/GM with excellent biocompatibility[11]. (c) GelMA gel coated on PCL/Gel nanofibers for tissue engineering scaffolds[57] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
(吕东, 杨琥, 郭学锋, 程镕时. 化学进展, 2010, 22(05):948.)
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
(李泓, 张静, 陈可, 罗程严, 徐春波, 梁宸, 李波, 肖文谦, 廖晓玲. 复合材料学报, 2020, 37(10):2572.)
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
(于靖, 孙莺, 王鹏, 陈鹏, 张永刚. 高分子通报, 2019, (5):1.)
|
| [51] |
(马颖, 王建全, 李向梅, 邵自强. 高分子材料科学与工程, 2021, 37(10):137.)
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
(殷星, 王璇, 马洪洋. 中国材料进展, 2019, 38(5):457-463.)
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
/
| 〈 |
|
〉 |