

Synthesis and Ring-Opening Metathesis Polymerization of Bio-Based Cyclic Olefins
Received date: 2024-03-22
Revised date: 2024-05-26
Online published: 2024-06-30
Supported by
National Key R&D Program of China(2019YFA0905000)
National Natural Science Foundation of China(22278223)
National Natural Science Foundation of China(22278205)
R&D Program of China Petrochemical Corporation (SINOPEC)(30000000-23-ZC0607-0871)
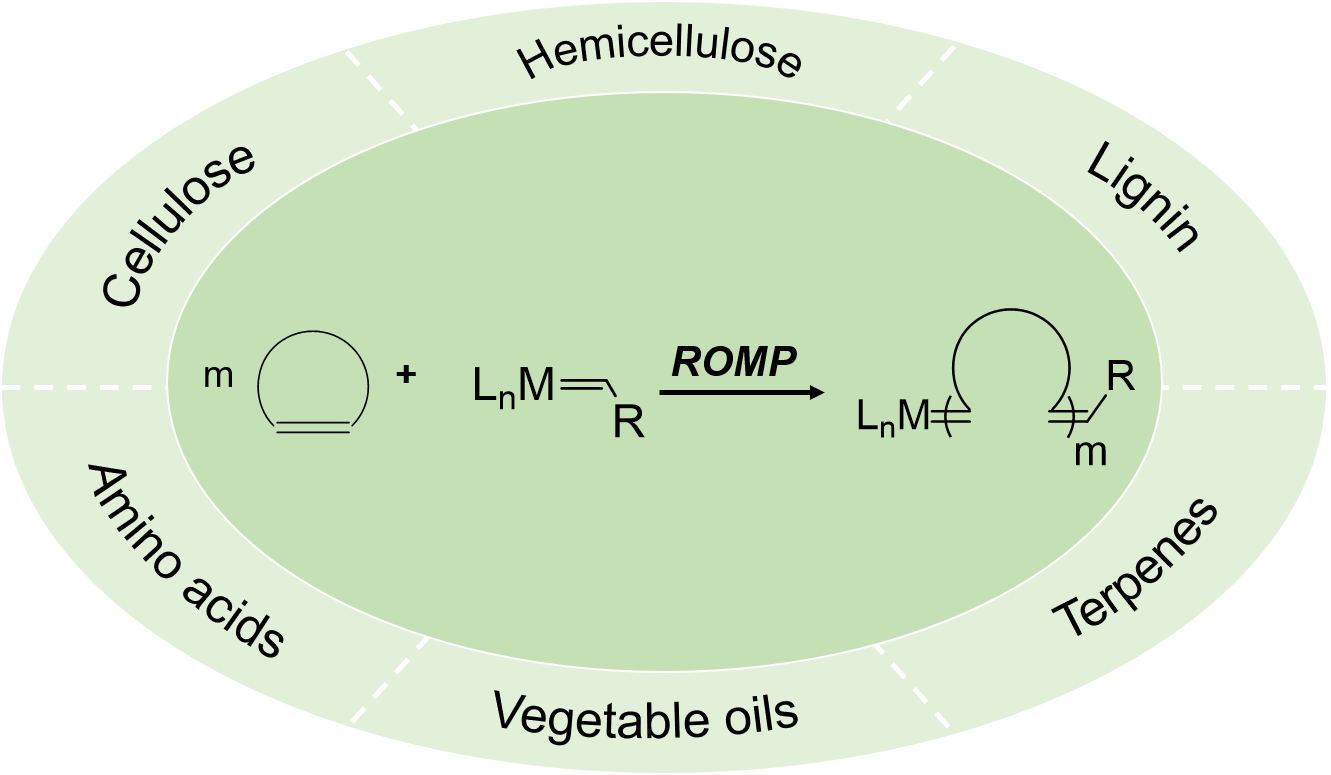
The transformations of biomass into bio-based polymeric materials have attracted growing interest from chemistry and material engineering. Ring-opening metathesis polymerizations (ROMP) of cyclic olefins have been identified as the powerful toolbox for synthesis of polyolefins containing double bonds in the polymer mainchains. Recently, a series of novel cyclic olefins are designed by using biomass as the feedstock, and high-performance polyolefins are prepared via ROMP of biomass derived monomers. This review summaries the advances in conversions of cellulose, hemicellulose, lignin, terpenes, vegetable oils, amino acids into norbornene derivatives, oxanorbornene derivatives, cyclooctene derivatives, macrocyclic olefins, etc. Synthesis and properties of bio-based polyolefins via ROMP of biomass derived monomers mentioned above are highlighted. Moreover, the challenges and opportunities are discussed with the aim to promote the development of bio-based polymeric materials.
1 Introduction
2 Cellulose-based cyclic olefins and ROMP
3 Hemicellulose-based cyclic olefins and ROMP
4 Lignin-based cyclic olefins and ROMP
5 Terpenes-based cyclic olefins and ROMP
6 Vegetable oils-based cyclic olefins and ROMP
7 Amino acids-based cyclic olefins and ROMP
8 Conclusion and outlook
Guangyu Pan , Xin Hu , Jie Yin , Yihuan Liu , Kai Guo , Ning Zhu . Synthesis and Ring-Opening Metathesis Polymerization of Bio-Based Cyclic Olefins[J]. Progress in Chemistry, 2024 , 36(12) : 1956 -1971 . DOI: 10.7536/PC240323
图11 呋喃基环烯烃的合成与ROMP: (a) endo-酯功能化内酯型三环氧杂降冰片烯[89-90]; (b) endo-酰胺功能化内酯型三环氧杂降冰片烯[91]; (c) 酯功能化内酯型三环氧杂降冰片烯单体[92]; (d) 酯功能化内酰胺型三环氧杂降冰片烯[93-94]Fig. 11 Synthesis and ROMP of furan-based cyclic olefins (a) endo-tricyclic oxa-norbornene lactone esters[89-90]; (b) endo-tricyclic oxa-norbornene lactone amides[91]; (c) Tricyclic oxa-norbornene lactone esters[92]; (d) Tricyclic oxa-norbornene lactam esters[93-94] |
图20 降冰片烯官能化植物油与交联剂的结构Fig. 20 Structures of norbornene functionalized vegetable oils and cross linkers |
表1 通过ROMP合成的植物油基热塑性材料的性能Table 1 Properties of vegetable oil-based thermosets |
| Sample | Tg (°C) | E (MPa) | εb (%) | E’ (MPa) | Ref |
|---|---|---|---|---|---|
| Dil100 | -29 | / | / | / | 111 |
| Dil60/DCPD40 | 21 | 0.91 | 138 | 10 | 110 |
| Dil50/DCPD50 | 39 | 27 | 132 | 187 | 110 |
| Dil40/DCPD60 | 58 | 68 | 156 | 799 | 110 |
| Dil30/DCPD70 | 76 | 525 | 35 | 1769 | 110 |
| NCO100 | -17 | / | / | 2.4 | 112 |
| NCO80/NCA20 | -6 | / | / | 5.7 | 112 |
| NCO60/NCA40 | 14 | 11 | 16.3 | 27.8 | 112 |
| NCO40/NCA60 | 27 | 25.7 | 25.2 | 130 | 112 |
| NCO20/NCA80 | 49 | 166.6 | 15.6 | 583.4 | 112 |
| NCA100 | 65 | 407 | 13 | 831.9 | 112 |
| NCA50/DCPD50 | 102 | 1699 | 14 | 1455* | 113 |
| NCA50/CL50 | 134 | 2051 | 8.8 | 2046* | 113 |
| PNESO | 66 | / | 27.9 | / | 114 |
E=Young’s modulus; εb=elongation at break; E’=Storage modulus (at 25 oC),* E’ at 30 ℃ |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
(马晓振, 罗清, 秦冬冬, 陈景, 朱锦, 颜宁. 化学进展, 2020, 32(5):617.).
|
| [12] |
(乔颖, 腾娜, 翟承凯, 那海宁, 朱锦. 化学进展, 2018, 30(9):1415.).
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
(郝杰, 高玉霞, 陈厚睿, 胡君, 巨勇. 高分子学报, 2020, 51(3): 239.).
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
(黄卫军, 朱宁, 方正, 郭凯. 化学进展, 2018, 30(12):1836.).
|
| [21] |
|
| [22] |
|
| [23] |
(袁鹏俊, 洪缪. 高分子学报, 2019, 50(4):327.).
|
| [24] |
|
| [25] |
|
| [26] |
(秦玉升. 功能高分子学报, 2019, 32(5): 558.).
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
(冯雨晨, 介素云, 李伯耿. 化学进展, 2015, 27(8):1074.).
|
| [45] |
(周楚璐, 侯翠苹, 陈伟, 王立杰, 程建华. 化学学报, 2022, 80(02): 229.).
|
| [46] |
|
| [47] |
|
| [48] |
(陈柯睿, 胡欣, 邱江凯, 朱宁, 郭凯. 化学进展, 2020, 32(1):93.).
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
(张浩伟, 韩文鉴, 刘一寰, 胡欣, 朱宁, 郭凯. 高分子学报, 2023, 54(11):1663.).
|
| [54] |
(陶永鑫, 陈蕾蕾, 刘一寰, 胡欣, 朱宁, 郭凯. 高分子学报, 2022, 53(12):1445.).
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
(杨佳鑫, 司传领, 刘坤, 刘华玉, 李晓云, 梁敏. 林业工程学报, 2020, 5(5):21.).
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
(李茂盛, 陈金龙, 陶友华. 应用化学, 2020, 37(3):280.).
|
| [126] |
|
/
| 〈 |
|
〉 |