

Precision Detection of Tumor Small Extracellular Vesicles
Received date: 2024-03-11
Revised date: 2024-06-21
Online published: 2024-06-30
Supported by
National Natural Science Foundation of China(22274077)
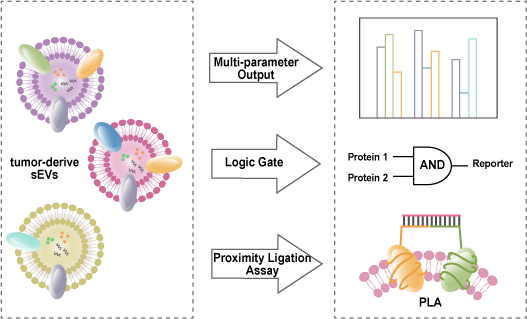
Tumor small extracellular vesicles (sEVs) are membranous vesicles, released by tumor cells, with a particle size less than 200 nm. They carry diverse biomolecular information on their surface and inside, participating in intercellular communication and are recognized as one of the most crucial liquid biopsies for cancer. Because sEVs’ surface contains a variety of proteins that can bind to corresponding antibodies or nucleic acid aptamers, quantitative detection of sEVs can be achieved through optical or electrochemical methods. However, due to the high heterogeneity and complexity of sEVs, relying on a single protein for recognition may lead to false positive or false negative signals. Therefore, accurate detection of tumor-derived sEVs requires simultaneous analysis of multiple biomarkers. Simultaneous analysis of multiple biomarkers can effectively address interference caused by phenotypic heterogeneity in sEVs and provide more accurate guidance for cancer diagnosis and prognosis. This paper focuses on the detection methods of sEVs based on surface proteins using fluorescence, colorimetry, electrochemical methods, and electrochemiluminescence techniques. It emphasizes the importance of achieving high sensitivity and accuracy in detecting sEVs through multi-protein multi-signal proportional output approaches, employing multi-protein logic gates and multi-protein proximity linking reactions.
1 Introduction
2 General analysis method of small extracellular vesicles
2.1 Fluorometry
2.2 Colorimetry
2.3 Electrochemical method
2.4 Electrochemical luminescence
3 Precision detection of small extracellular vesicles based on surface multiproteins
3.1 Multiprotein proportional output
3.2 Multiprotein Logical Computation Output
3.3 Multiprotein proximity ligation reaction
4 Conclusion and outlook
Huijing Wang , Die Sun , Ruiying Wang , Hui Zhang . Precision Detection of Tumor Small Extracellular Vesicles[J]. Progress in Chemistry, 2024 , 36(12) : 1972 -1982 . DOI: 10.7536/PC240318
图7 用于小细胞外囊泡分析的多参数输入逻辑门。(a)小细胞外囊泡三种表面蛋白对应的核酸适配体。(b)核酸适配体结合小细胞外囊泡的AND逻辑门检测系统,用于处理基于EpCAM、CD63和PTK7三种蛋白的分子信号。只有当所有目标蛋白都存在时,才能释放初始信号进行等温扩增[86]Fig. 7 Multi-parameter input logic gate for small extracellular vesicles analysis. (a) Nucleic acid aptamers corresponding to three surface proteins of small extracellular vesicles. (b) The AND logic gate detection system of aptamer binding to small extracellular vesicles is used to process molecular signals based on EpCAM, CD63 and PTK7 proteins. Only when all the target proteins are present can the initial signal be released for isothermal amplification[86]. Copyright 2022, American Chemical Society |
图8 基于核酸适配体的邻近连接反应高灵敏定量检测肿瘤来源外泌体的工作原理。利用设计的两种分别针对EpCAM和PD-L1的核酸适配体探针同时标记小细胞外囊泡上的两种蛋白标记物。由于外泌体膜的流动性,两个核酸适配体的延伸部分接近。连接后,使用dd PCR对连接产物进行定量[96]Fig. 8 Working principle of the highly sensitive quantification of tumor-derived Exo-PD-L1 using aptamer-based PLA. Two types of designed aptamer probes against EpCAM and PD-L1 were utilized to simultaneously label both types of protein markers on the exosomes. The extended parts of two aptamers are in close proximity because of the fluidity of the exosomes membrane. After ligation, ddPCR is performed to quantify the ligation products [96]. Copyright 2020, Wiley Online Library |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
/
| 〈 |
|
〉 |