

Fabrication and Biomedical Application of Hyaluronic Acid Based Micro- and Nanogels
Received date: 2022-10-09
Revised date: 2023-01-11
Online published: 2023-02-20
Supported by
National Key Research and Development Project(2020YFE0204400)
National Natural Science Foundation of China(51873186)
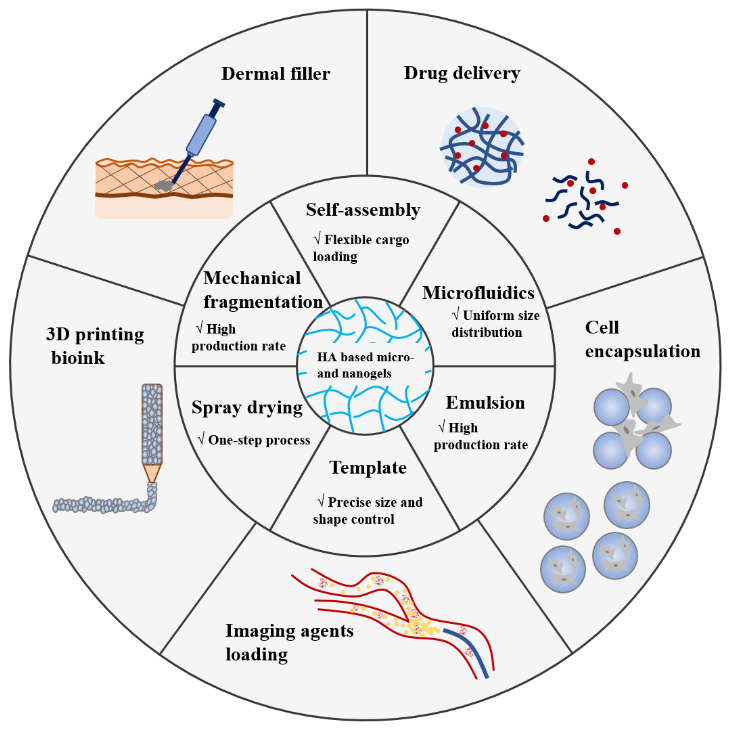
Hyaluronic acid is one of the primary components of the natural extracellular matrix, which has outstanding biocompatibility, biodegradability and excellent water retention capacity. Hyaluronic acid based micro- and nanogels have many desirable features of size adjustability, easy modification, deformability and structural integrity. They can not only meet most application requirements of bulk hydrogels, but also possess unique advantages due to small dimension, thus have been widely researched and used in biomedical field. This paper reviews the preparation methods and biomedical applications of hyaluronic acid based micro- and nanogels, and the future development of it is prospected.
Xuedan Qian , Weijiang Yu , Junzhe Fu , Youxiang Wang , Jian Ji . Fabrication and Biomedical Application of Hyaluronic Acid Based Micro- and Nanogels[J]. Progress in Chemistry, 2023 , 35(4) : 519 -525 . DOI: 10.7536/PC221001
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
/
| 〈 |
|
〉 |