

Heterogeneous Bifunctional Catalysts for Catalyzing Conversion of Levulinic Acid to γ-Valerolactone
Received date: 2022-09-26
Revised date: 2022-11-10
Online published: 2023-02-20
Supported by
National Natural Science Foundation of China(51906084)
Program for Taishan Scholars of Shandong Province Government
R&D program of Shandong Basan Graphite New Material Plant and Innovation
Entrepreneurship Training Program for College Students of Shandong Province(S202110427093)
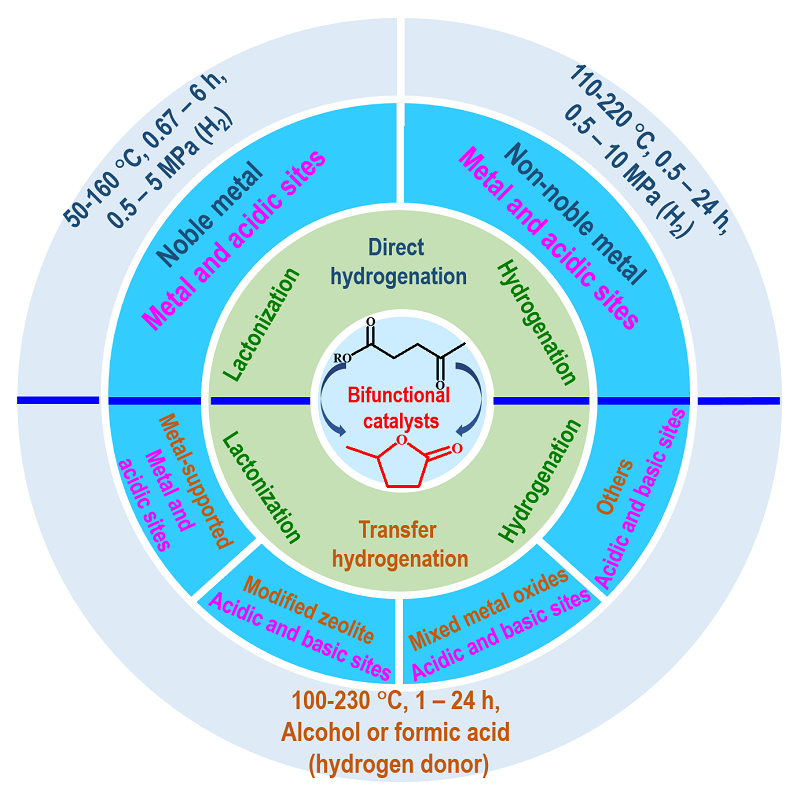
Levulinic acid is important biomass-derived compounds, and catalytic conversion of them to γ-valerolactone (GVL) over heterogeneous bifunctional catalysts has become a hot focus in the field of biorefining. In this paper, the direct hydrogenation of levulinic acid and its esters to GVL catalyzed by noble and non-noble metal bifunctional catalysts, and the transfer hydrogenation of levulinic acid and its esters to GVL catalyzed by the bifunctional catalysts, such as metal-supported catalysts, modified zeolite, and mixed metal oxides, are reviewed. Conversion of levulinic acid and its esters to GVL over bifunctional catalysts involves two steps, including hydrogenation of carbonyl group and subsequent lactonization reaction. In addition, the importance of active sites of various bifunctional catalysts in conversion of levulinic acid and its esters is studied in this paper, and the advantages and problems of different catalysts during the conversion of levulinic acid/esters are discussed. Finally, the development of bifunctional catalysts and the synthesis of GVL in the future are prospected.
Yuewen Shao , Qingyang Li , Xinyi Dong , Mengjiao Fan , Lijun Zhang , Xun Hu . Heterogeneous Bifunctional Catalysts for Catalyzing Conversion of Levulinic Acid to γ-Valerolactone[J]. Progress in Chemistry, 2023 , 35(4) : 593 -605 . DOI: 10.7536/PC220928
表1 不同贵金属双功能催化剂催化转化乙酰丙酸及其酯制备γ-戊内酯Table 1 Conversion of levulinic acid and its esters to γ-valerolactone catalyzed by different noble metal bifunctional catalysts |
| Entry | Catalysts | T (℃) | (MPa) | t (h) | Solvent | Con. (%) | Yield (%) | ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Ru/rGO | 50 | 2.0 | 0.67 | H2O | 100.0-LA | 18.0 | 22 |
| 2 | Ru/rGO-S | 50 | 2.0 | 0.67 | H2O | 100.0-LA | 82.0 | 22 |
| 3 | Ru/C + A70 | 70 | 0.5 | 3 | H2O | 98.0-LA | 98.0 | 23 |
| 4 | Ru/OMC-P | 70 | 0.7 | 6 | H2O | 98.0-LA | 92.1 | 24 |
| 5 | Ru/HfO2@CN | 80 | 1 | 3 | H2O | 100.0-LA | 92.0 | 25 |
| 6 | Ru/N@CNTs | 80 | 1 | 1 | H2O | 100.0-LA | 99.0 | 26 |
| 7 | Ru/DOWEX | 70 | 1 | 4 | H2O | 98.3-LA | 98.0 | 27 |
| 8 | Ru/MCM-49(DP) | 160 | 2.5 | 0.5 | H2O | 94.3-LA | 93.4 | 28 |
| 9 | Ru-Mn(0.7)/MCM-49 | 160 | 2.5 | 3 | H2O | 98.0-LA | 98.0 | 29 |
| 10 | Ru/Zr10SMS | 70 | 0.5 | 3 | H2O | 98.4-LA | 94.5 | 30 |
| 11 | Ru/SMS | 70 | 0.5 | 3 | H2O | 99.2-LA | 95.6 | 30 |
| 12 | Ru/(AlO)(ZrO)0.1 | 120 | 1 | 6 | H2O | 100.0-LA | 100.0 | 31 |
| 13 | Ru/NbOPO4/SBA-15a | 100 | 1 | - | H2O | 100.0-LA | 86.0 | 32 |
| 14 | Ru/TiO2 | 70 | 5 | 1 | H2O | 100.0-LA | 100.0 | 33 |
| 15 | Ru/MIL-101(Cr) | 70 | 1 | 5 | H2O | 100.0-LA | 99.0 | 34 |
| 16 | Ru/SPES | 70 | 3 | 2 | H2O | 87.9-LA | 87.9 | 35 |
| 17 | Ru/HAP | 70 | 0.5 | 4 | H2O | 99.0-LA | 99.0 | 36 |
| 18 | Pd/HAP | 70 | 0.5 | 4 | H2O | 26.0-LA | 23.4 | 36 |
| 19 | Pd/CeO2 | 90 | 0.4 | 1.5 | 2-PrOH | 100.0-LA | 99.9 | 37 |
| 20 | Pd@ND | 150 | 0.5 | 12 | H2O | 100.0-LA | 96.0 | 38 |
| 21 | Pd@mSiO2 | 200 | 3.0 | 4 | dioxane | 95.0-LA | 91.2 | 39 |
| 22 | 5 wt% Pd/MCM-41 | 240 | 6.0 | 10 | H2O | 100.0-LA | 96.3 | 40 |
| 23 | Pt/HAP | 70 | 0.5 | 4 | H2O | 42.0-LA | 37.0 | 36 |
| 24 | Pt/Y-C18TAOH | 120 | 2.5 | 6 | H2O | 100.0-LA | 94.0 | 41 |
aHydrogenation of levulinic acid on a fixed bed reactor |
表2 不同非贵金属双功能催化剂催化转化乙酰丙酸及其酯制备γ-戊内酯Table 2 Conversion of levulinic acid and its esters to γ-valerolactone catalyzed by different non-noble metal bifunctional catalysts |
| Entry | Catalysts | T (℃) | (MPa) | t (h) | Solvent | Con. (%) | Yield (%) | ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Ni/Al2O3-CN-600 | 130 | 0.5 | 3 | THF | 100.0-LA | 99.0 | 42 |
| 2 | Ni@C | 200 | 3 | 4 | Dioxane | 100.0-LA | 100.0 | 43 |
| 3 | Ni/C-500 | 200 | 1 | 5 | Dioxane | 100.0-LA | 98.2 | 44 |
| 4 | Ni-Mo/C | 200 | 10 | 2 | Dioxane | 100.0-LA | 100.0 | 45 |
| 5 | Ni/H-ZSM-5a | 320 | - | - | - | 98.6-LA | 98.6 | 46 |
| 6 | Ni/Al2O3 | 200 | 5 | 4 | 2-PrOH | 92.0-LA | 92.0 | 47 |
| 7 | Ni/MgO-Al2O3 | 160 | 3 | 1 | Dioxane | 99.7-LA | 99.7 | 48 |
| 8 | Ni/SiO2-Al2O3 | 200 | 1.58 | 0.5 | THF | 100.0-LA | 100.0 | 49 |
| 9 | Ni-Al | 170 | 5 | 2 | H2O | 100.0-LA | 99.0 | 50 |
| 10 | CeNi/Si | 275 | AT. | - | - | 82.5-LA | 79.3 | 51 |
| 11 | Ni(OAc)2·4H2O/DPPP | 180 | 1 | 10 | Free | 100.0-LA | 95.1 | 52 |
| 12 | Co@NC-700 | 190 | 1.9 | 2 | Dioxane | 100.0-LA | 100.0 | 53 |
| 13 | Co/Ya | 200 | - | - | - | 99.0-LA | 80.0 | 54 |
| 14 | Co-LA@SiO2-800 | 120 | 3 | 24 | Dioxane | 100.0-LA | 96.0 | 55 |
| 15 | Co/SiO2(8.1)a | 200 | 3 | - | - | 100.0-EL | 98.0 | 56 |
| 16 | Co-Al | 150 | 3 | 2 | H2O | 100.0-EL | 98.0 | 57 |
| 17 | 2.1Co-0.9Mg-Al | 150 | 3 | 2 | H2O | 100.0-EL | 97.0 | 57 |
| 18 | Cu/Zr-Al-3 | 170 | 3 | 5 | H2O | 100.0-LA | 100.0 | 58 |
| 19 | CuAl | 110 | 3 | 2 | Ethanol | 100.0-LA | 95.3 | 59 |
| 20 | 3Cu/Zr0.8-C | 260 | 0.5 | - | - | 88.5-LA | 83.4 | 60 |
| 21 | Ni/Cu/Al/Fe | 150 | 5 | 3 | Methanol | 100.0-LA | 99.0 | 61 |
| 22 | Ni4.59Cu1Mg1.58Al1.96Fe0.70 | 142 | 2 | 3 | Methanol | 100.0-LA | 98.1 | 62 |
| 23 | Ni2Co1P | 180 | 3 | 4 | Free | 100.0-LA | 100.0 | 63 |
| 24 | Cu-Ni/Al2O3-ZrO2 | 220 | 3 | 0.33 | 2-Butanol | 100.0-LA | 99.9 | 64 |
| 25 | Cu-Ni/Al2O3 | 180 | 2.5 | 6 | Ethanol | 99.0-EL | 97.0 | 65 |
aHydrogenation of levulinic acid on a fixed bed reactor |
表3 不同双功能催化剂催化乙酰丙酸及其酯转移加氢制备γ-戊内酯Table 3 Transfer hydrogenation of levulinic acid and its ester to γ-valerolactone catalyzed by different bifunctional catalysts |
| Entry | Catalysts | T(℃) | t (h) | Solvent | Con. (%)a | Yield (%) | ref |
|---|---|---|---|---|---|---|---|
| 1 | Ru/g-C3N4 | 100 | 12 | 2-Propanol | 100.0-LA | 99.8 | 66 |
| 2 | Ru(OH)x/TiO2 | 90 | 24 | 2-Propanol | 100.0-ML | 80.0 | 67 |
| 3 | Ni/E-cats | 180 | 6 | 2-Propanol | 90.3-EL | 86.9 | 68 |
| 4 | CuNi-0.4Al/AC | 220 | 2 | 2-Propanol | 100.0-LA | 97.2 | 69 |
| 5 | Ni3P-CePO4(0.1) | 180 | 2 | 2-Propanol | 99.9-LA | 89.9 | 70 |
| 6 | Ni/ZrO2 | 100 | 20 | 2-Propanol | 100.0-ML | 94.0 | 71 |
| 7 | Cu/AC | 200 | 7 | 2-Propanol | 100.0-LA | 89.9 | 72 |
| 8 | Hf@CCSO3H | 200 | 24 | 2-Propanol | 100.0-LA | 96.0 | 73 |
| 9 | Zr-beta | 118 | 10 | 2-Propanol | 100.0-LA | 96.0 | 74 |
| 10 | Zr-Al-Beta | 170 | 24 | 2-Propanol | 100.0-LA | 85.5 | 75 |
| 11 | SnO2/SBA-15 | 110 | 8 | 2-Propanol | 85.0-LA | 80.8 | 76 |
| 12 | GluPC-Zr | 190 | 12 | 2-Propanol | 100.0-LA | 98.1 | 77 |
| 13 | ZrO2(10)/SBA-15 | 150 | 3 | 2-Propanol | 100.0-LA | 90.0 | 78 |
| 14 | ZrFeO(1:3)-300 | 230 | 3 | Ethanol | 100.0-EL | 87.2 | 79 |
| 15 | ZrO2 | 150 | 16 | 2-Butanol | 100.0-LA | 92.0 | 80 |
| 16 | Zr1Fe1-150 | 200 | 1 | 2-Propanol | 100.0-LA | 96.7 | 81 |
| 17 | Mn2CoOx | 230 | 9 | Formic acid | 80.0-LA | 77.0 | 82 |
| 18 | ZrF MOFs | 200 | 2 | 2-Propanol | 98.0-LA | 96.0 | 83 |
| 19 | Zr-humic acids | 150 | 3 | 2-Propanol | 96.4-EL | 75.8 | 84 |
| 20 | UiO-66-S60 | 140 | 24 | 2-Butanol | 98.0-ML | 82.0 | 85 |
| 21 | HPW@MOF-808 | 160 | 6 | 2-Propanol | 100.0-LA | 87.0 | 86 |
aLA: Levulinic acid; ML: methyl levulinate; EL: ethyl levulinate. |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
/
| 〈 |
|
〉 |